中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
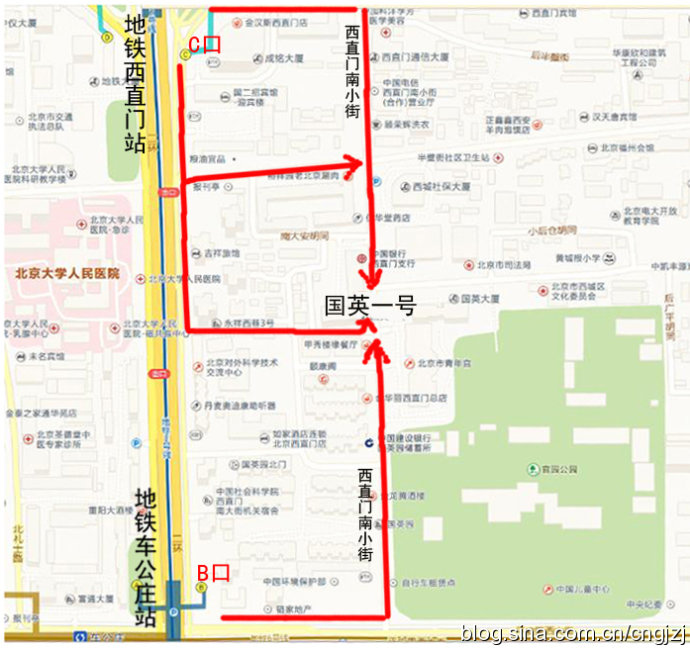
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
2013年01月06日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Traditional Chinese medicine research and development achievements four big problem
On December 5, 2012
Source: Beijing commercial daily
From the 16 th Beijing international biological medicine industry development on the BBS's point of view, according to the traditional Chinese medicine research and development achievements in research and development achievements and disconnected from the market, enterprise scientific research funds investment insufficiency, the interest distribution rules don't mature, new drug approval four questions too low throughput rate, to a certain extent, restricted the development of the industry.
Herbal treatment not disease has unique advantages, as the major health market fast development, traditional Chinese medicine research and development is imminent. Experts point out that the adjustment of industrial policy and so on will help reduce the difficulty of new born.
Research market maturity
There are still many scientific research institutions system, a lot of research and development personnel don't study the market
New drugs often because of its good performance in the market will make a lot of enterprises under the net cost, but in the last weekend of the 16 th Beijing international biological medicine industry development on the BBS, Dr, Peking University health science HanJingYan that hospital nepp's, institute of Chinese medicine and so on all is the subject of scientific research, the development of new medicine by the power of the enterprise is very difficult to complete, need the support of scientific research institutions. Good co-operative mode is the foundation of new drug research and development.
Market economy led to many scientific research institutions to adapt to the market demand, but at present there are still many scientific research institutions system, a lot of research and development personnel not market research, the results just to devote, to get the degree, title, etc. A lot of innovation is to achieve "self", this kind of mentality in traditional Chinese medicine research world wide. Under the research and development of the ecological environment, many eager for new drugs of traditional Chinese medicine enterprise think development of new products and market demand. "We want to cooperate with medical research institute to develop new products, but the development of scientific research institutes low product market maturity, it is difficult to into goods. In addition, the research and development achievements low maturity and scientific research institutions for low also has a lot to do." Ridge pharmaceutical (002603 strands of) directors TianShuYan on the BBS, for example, more than 301 hospital of Beijing scientific research funds, the hospital scientific research personnel to develop products of high maturity, cooperation prospects is very good. It is reported that 301 hospital for funds investment of scientific research every year for 100 million - 200 million yuan, and local hospital it will be difficult to obtain funds support so much.
Interest distribution rules it is not clear
Some research institutions even hope to be able to get 50% of the new drug sales
Research and development institutions and the achievements of the enterprise how to into is transformation of scientific research of traditional Chinese medicine (TCM) is a big problem. Scientific research institutions in the new drugs listed before is just take patent rights transfer, or participate in sales into new drugs, this rule in the industry at present is not sure.
"From the new drug research and development to the market, the workload is about 10% for scientific research institutions, the workload is 90% of the enterprise, if undertake profit allocation according to workload, new drug sales 10% of revenue to the scientific research institutions." TianShuYan is introduced. It is understood that in a foreign country, scientific research institutions and enterprises of the divided into 5% : 95%.
"Rules of the game is not clear, scientific research institutions to work return expectations too high, some scientific research institutions hope to be able to get new medicines 50% of sales amount, it is difficult to achieve the cooperation between research institutions and enterprises is an important reason." TianShuYan said.
Not only enterprises and research institutions, scientific research personnel and research institutions is a a mess. As early as in 2007, hebei medical university professor LiEnFu charged with traditional Chinese medicine "MaLuoDan" inventor identity handan pharmaceutical limited company, and hebei province hospital claimed that "MaLuoDan" intellectual property right shall belong to the hospital. Hebei institute of traditional Chinese medicine medical research director CaoDongYi analysis, scientific research personnel of labor points position and - labor, if in his spare time to finish the invention, the invention belongs to the inventor of intellectual property rights; If it is in the work time of product development, the invention of intellectual property rights to scientific research institutions, but at present the work of scientific research personnel and it is difficult to define in your spare time.
Enterprise scientific research investment funds too little
Foreign r&d investment in the sales accounted for about 15%, the domestic enterprise of only 1%
Scientific research institutions for less, go against the development of new drugs, enterprise scientific research funds shortage but also problems. So enterprise for scientific research of the how much money of total sales?
In the 16 th Beijing international biological medicine industry development on the BBS HanJingYan asked this question, the answer is: Beijing north viction biological science and technology limited company is 10%, tong (600085, shares) group was 3%, the ridge pharmaceutical is 3%, nine zhi hall of a joint stock limited company is 1%. In foreign enterprise investment in research and development costs accounted for 15% of total sales.
Obviously, the domestic enterprises for the cost of new drug research and development is very few, this is bad for the birth of new drugs.
Traditional Chinese medicine treatment of disease has unique advantages. With the development of the health industry, the cost of the enterprise for research and development will also be increased accordingly. Beijing and jun consulting company limited partners LiWenMing told reporters that if research in place, now the health industry output value reached one trillion yuan easily. He of yunnan baiyao (000538, shares) yunnan baiyao toothpaste group, from Colgate and crest, grab a slice cosmetic mogul of the case said.
Tong health pharmaceutical co., LTD., vice general manager ZhangHong said: "Chinese medicine medicine food homology, tongren temple in the production of cosmetics, health products, daily necessities, and so on profit margin on the rise. Amway in China's sales reached 10 billion yuan, to transcend the number."
Research and development of new drug approval rate is low
New drug approval passing rate is very low, attrition rate of 80%
Starting from new drug research and development, to the state food and drug administration approved, it will take 7 to 8 years of time, this time some scientific research institutions is can't afford the and so on. And new drug approval rate is very low, the attrition rate of 80%.
CaoDongYi talking about their new drug research and development, it is not without regret, he used his spare time and shijiazhuang 5 pharmaceutical factory cooperation, develop famous 士达 oral liquid, 1996 approved by the ministry of health "jian" name. Obtained from research to the batch number, spent four years time.
However, the notable celebrities of oral liquid fate, are catching up with the factory and north China pharmaceutical (600812, shares) enterprise merger, after the cooperation of the industry of the invention without doing any market promotion, so CaoDongYi years of painstaking effort into water. "Although didn't get the patent rights transfer, I will not say what, this company had spent hundreds of thousands of yuan, the product is used for clinical trials erase any wealth is equal to wasting resources." CaoDongYi appears very helpless. Although it is the case, it shows that industry rules of risk control and prevention is not clear.
HanJingYan thinks that the development of TCM products industry policy to make proper adjustment, but due to the development and achievements respectively by the national development and reform commission, ministry of science and technology management department, each department starting point is different, so in politics than out, coordinate is difficult, so the new industrial policy is not overnight thing, need to consider the actual situation of industry and research.
Business newspaper reporter LiuYaLi