中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
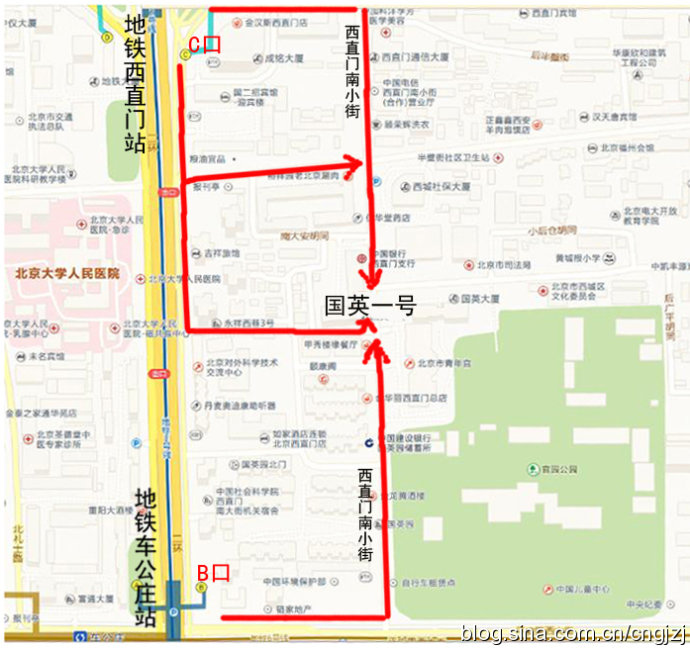
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2010年12月07日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Technical requirements for cosmetic
products on the issuance of the notification specification
State Food and Drug Administration Xu [2010] 454
November 26, 2010 release
Provinces, autonomous regions and municipalities Food and Drug Administration (SDA):
To further standardize the administrative license cosmetics, raise the level of quality and safety control cosmetics, cosmetic production and management to strengthen health surveillance, technical requirements guiding the preparation of cosmetic products, the State Food and Drug Administration formulated the "technical requirements for cosmetic products specifications", is hereby issued, please follow.
Annex: 1. State Food and Drug Administration cosmetic product technical requirements (text format)
2. Guidelines for the preparation of cosmetics products, technical requirements
State Food and Drug Administration
二○一 ○ 年 十一月 二十 六日
Cosmetic product technical requirements specification
First, under the "Health Supervision of cosmetics", to further standardize the administrative license cosmetics, raise the level of quality and safety control cosmetics, cosmetic production and management to strengthen health surveillance, protection of consumer safety, the development of this specification.
Second, the State Food and Drug Administration approved the cosmetic product is responsible for technical requirements, and monitor its implementation.
Third, technical requirements for cosmetic products must conform to the relevant laws, regulations, standards and norms.
Fourth, technical requirements for cosmetic products should be consistent with the provisions of its text format. Text should include the product name, recipe ingredients, production process, sensory indicators, chemical indicators of health, microbial indicators, test methods, instructions for use, storage conditions and shelf life sequence (see Annex 1), and in accordance with the "technical requirements for cosmetic products Guidelines "(see Annex 2) prepared.
Fifth, technical requirements for cosmetic products, health quality and safety of the product technical support. Manufacturers of cosmetics products in accordance with technical requirements should organize production and management, food and drug supervision and management departments technical requirements for cosmetic products should be carried out as an important basis for health supervision and law enforcement.
Sixth, the technical requirements for cosmetic products, cosmetic products, new product licensing and renewal.
Seven technical requirements for cosmetic products number. Special use cosmetics made in accordance with HZ + GT + Year +0000 preparation; imports of cosmetics for special purposes in accordance with HZ + JT + Year +0000 preparation; imported non-special use cosmetics in accordance with the HZ + JF + Year +0000 establishment. "HZ" means "cosmetic", "GT" means "domestic special purpose", "JT" that "imported special purpose", "JF" that "imports of non-special purpose", "Year +0000" for the approval of the text of cosmetics ( or record number) of the year and sequence number.
Eight of the specification since April 1, 2011 shall come into force.
Annex 1
State Food and Drug Administration
Technical requirements for cosmetic products (text format)
(Product technical requirements No.)
_______________________________________
Chinese name
Pinyin name
Recipe ingredients】 【
】 【Production
【Index】 sensory
Chemical index】 【Health
】 【Microbe
【Test Method】
Usage
】 【Storage conditions
【Shelf life】
_______________________________________
Annex 2:
Guidelines for the preparation of cosmetics products, technical requirements
First, the main content
Technical requirements for cosmetic products, and control should be able to accurately reflect the quality and safety of health products. Technical requirements for cosmetic products, each of which shall meet the following requirements and cosmetic products in accordance with the specific requirements for disclosure of information compiled.
(A) Name
Including the Chinese name and Chinese pinyin name. Product name should be accurate, clear, to show that the product of real property, in line with "cosmetic naming rules."
(B) of the formula ingredients
The formula ingredients should include the production of all raw materials used in products. All material should be content in descending order, and indicate its intended use. Cosmetics raw materials used should meet the "Hygienic Standard for Cosmetics," the relevant requirements.
Formula composition of materials used in the Chinese name should use "international standards of the Chinese name of the directory of cosmetics raw materials" standard Chinese name. Name of raw materials without international cosmetics (INCI name) or not included in the "standards of international cosmetics raw materials Chinese name directory", use "Chinese Pharmacopoeia" in the name or chemical name or Latin name of plants.
(C) of the production process
Brief description of the complete application of text production process.
(D) Sensory Index
The contents were the product should be the color, character, odor and other indicators in order to describe the sensory and separated by semicolons.
(E) chemical indicators of health
(F) microbial indicators
Sensory indicators, chemical indicators of health, microbial indicators and other relevant content should be described under the "cosmetic management of administrative license test," and so the requirements identified test items, index. If you use the table to provide information beneficial understanding of test items, are advised to use the list.
(G) testing method
Chemical indicators should be health, microbial indicators of test methods were listed.
(H) use
Use of cosmetics should be elaborated and precautions.
(Ix) storage conditions
Product packaging and product should be based on stability characteristics of their products, storage conditions described, such as temperature, away from light and so on.
(X) durability
The results should be determined according to the relevant product shelf life, shelf life of the format should be labeled as: the production date and shelf life or production lot and the restricted use of date.
Second, the basic requirements
(A) of the preparation should be consistent with national laws, administrative regulations, departmental rules and regulations, technical standards and regulatory documents of the relevant regulations.
(B) the technical requirements of product design, content and data should be consistent with generally accepted scientific principles, accurate and reliable.
(C) technical requirements for products, text, numbers, formulas, units, symbols, diagrams, etc. should conform to standardized requirements, reference standards accurately and effectively. Definition of terms should be consistent with relevant state regulations.
1. Should use the standard Chinese characters. Punctuation should be consistent with the provisions of GB/T15834.
2. Should use the GB3101, GB3102 statutory units of measurement. Said the money should write their units.
3. Should be accurate reference standards listed in the directory or file.
4. Referenced standards or documents shall include the publication of this reign, and the complete number or standard (file) name.
5. If the reference standard (paper) available online to the Internet should provide detailed access and access paths. Should be given the referenced standard (document) the complete URL. To ensure traceability, should provide the source URL.
(D) Product technical requirements established in the detection method is accurate, precise, and after method validation.
(E) Product technical requirements have limited requirements, to use explicit numerical representation. Not only qualitative statements such as "moderate" or "proper temperature" and so on.
(Vi) Product technical requirements of the experimental records and writes must be true, complete, clear, pristine nature and traceable. Their research methods and processes to accurately record and report data to be fully realized.
(Vii) technical requirements for products used in the table should be explicitly mentioned in the article.
1. Does not permit the table in the table, the table does not permit further divided into sub-tables.
2. Each table should be numbered. Table number from the "table" and the Arabic numerals from 1 composition, such as "Table 1", "Table 2" and so on. Only one table, should give number "in Table 1."
3. Each table should have the table title.
4. Each table should have headers. Table column should generally be placed with the corresponding units of the table column header names of the amount under the header does not permit the use of slash.
5. If a connected row of the table need to switch pages, you then take the pages of the table row should repeat the table number, table title and "(continued)." Continued header and should be repeated statements on the unit.
(H) Product technical requirements may involve intellectual property rights, the state food and drug administration departments do not bear the responsibility to identify the intellectual property rights.
(Ix) should use the national standard recognized by the legal department of substances (including standards and standard). The use of contrast material is developed, and should be related to the identification required by the relevant research data and control substances. Samples for research should be the formula to determine, the production process is stable, representative batches of products.
(J) to carry out the technical requirements of products should meet the technical requirements of the product laboratory conditions, by the appropriate technical staff commitment.