中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
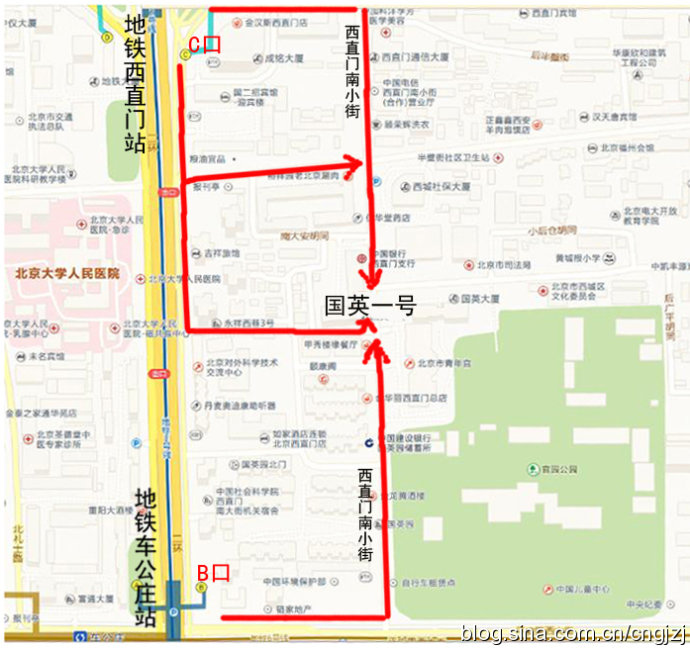
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Chinese medicine laws and regulations
您现在的位置是:
首页 >>
Regulations >>
Chinese medicine laws and regulations
2011年01月10日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Ministry of Health
State Administration of Traditional
State Food and Drug Administration
File
State Administration of Traditional Chinese Medicine Hospital issued 〔2010〕 39
Strengthen medical institutions on the issuance of
traditional Chinese medicine preparation management advice notice
Provinces, autonomous regions and municipalities health bureau, Traditional Chinese Medicine, Food and Drug Administration, Bureau of Xinjiang Production and Construction Corps, Health, Food and Drug Administration:
Chinese medicine preparations for medical institutions to meet the needs of the masses of this service, improve the clinical efficacy of traditional Chinese medicine to keep playing features and advantages of Chinese medicine, Chinese medicine to promote the inheritance and innovation of great significance. Traditional Chinese medicine preparation to strengthen management of medical institutions, medical institutions to promote the development of Chinese medicine preparations, the Ministry of Health, the State Pharmaceutical Administration and the State Food and Drug Administration jointly formulated the "on strengthening the management of medical institutions in the views of traditional Chinese medicine preparation," is hereby issued. Please follow throughout the implementation in practical work.
August 24, 2010
On strengthening the management of medical institutions in the views of traditional Chinese medicine preparation
Medical institutions, medical institutions, traditional Chinese medicine preparation is required under this unit of approved clinical preparation, personal use of the fixed-counter preparations of traditional Chinese medicine. Over the years, traditional Chinese medicine preparation in medical institutions to meet clinical demand, promote the development of Chinese medicine has played an important role, but there are also uneven development, combined with traditional Chinese medicine is not enough clinical needs, strengths and characteristics do not reflect the salient issues. According to "Drug Administration Law" and relevant regulations, to implement the "CPC Central Committee and State Council on Deepening the views of medical and health system" (in the [2009] 6) and "the State Council on the pharmaceutical industry to support and promote the development of a number of opinions" (Guo Fa [2009] 22), follow the law of development of Chinese medicine, traditional Chinese medicine preparation to fully embody the characteristics of traditional Chinese medicine preparation to strengthen management of medical institutions, medical institutions to promote the development of Chinese medicine preparations are made the following observations:
First, the profound understanding of the development of medical institutions the importance of Chinese medicine preparations
Medical institutions, clinical application of traditional Chinese medicine preparation to good Chinese medicine prescriptions based development, with clinical efficacy, ease of use, cost and other advantages of relatively low, reflecting the regional characteristics of traditional Chinese medicine, hospital characteristics, college characteristics and clinical experience of doctors is Chinese medicine important part of clinical medicine. Medical institutions to make use of Chinese medicine formulations lack of commercial proprietary products will help meet the needs of the masses of this service; to serve the clinical needs, helping improve the clinical efficacy of traditional Chinese medicine; can bring the characteristics of specialist and hospital characteristics of the construction and development and help keep the play features and advantages of Chinese medicine; to effectively inherit the name of the old Chinese medicine experts, clinical experience, is conducive to the inheritance and innovation of Chinese medicine; to lay a good foundation for the new drug development, and promoting traditional Chinese medicine drug discovery. "State Council on the pharmaceutical industry to support and promote the development of a number of opinions" (Guo Fa [2009] No. 22) that we should "encourage and support the development and application of medical institutions feature traditional Chinese medicine preparation." Medical institutions to support and promote the development of Chinese medicine preparations for the deepening of medical and health system, improving the people's health, and promote a harmonious society is very important.
Second, the development of medical institutions, the basic principles of Chinese medicine preparations
One important characteristics. The development of medical institutions to work closely with traditional Chinese medicine preparation of Chinese Medicine of the characteristics of medical institutions, focus reflects the characteristics of geographic features and disease spectrum, reflecting the process, formulation of the traditional characteristics and reasonable.
The second is about effectiveness. The development of medical institutions should pay attention to the safety of Chinese medicine preparations, highlighting the efficacy, quality assurance, ease of use, with the local economic and social development level.
Third, we must focus. The development of medical institutions to co-ordinate planning of Chinese medicine preparations, highlighting key areas and species, to avoid the blind pursuit of number of species, changes in small but complete, the status of many and scattered.
Fourth, re-transmission. Development of medical institutions to focus on traditional Chinese medicine preparation to old TCM prescription long-term clinical practice based on clinical experience with the name of the old traditional Chinese medicine and academic heritage combined.
Fifth, follow the law. The development of medical institutions should not only reflect the diagnosis and treatment of Chinese medicine preparations, highlighting the traditional characteristics of Chinese medicine, but also to follow the basic rules of drug development, focusing on the accumulation of clinical data and results of the evaluation.
Sixth, and development. The development of Chinese medicine formulations medical institutions should give top priority to social benefits, based on meeting the needs of patients, standardized management, and constantly improve the level of preparation, in the name of Branch, the name of hospital construction and development services in the pharmaceutical industry.
Third, strengthen the management of medical institutions registered Chinese medicine preparations
(A) of the provinces, autonomous regions and municipalities drug regulatory authorities should "Drug Administration Law," "Drug Administration Law Implementing Regulations" and other laws and regulations, strengthen supervision of medical management of traditional Chinese medicine preparation to ensure the safety of medical institutions, traditional Chinese medicine preparation , effective and quality control. Shall be in accordance with the "medical preparations Registration" (Trial) requirements, combined with the practical details of implementation of local, highlighting the tradition, reflecting the characteristics of Chinese medicine theory, Chinese medicine clinical advantages to play, to lay the foundation for Chinese medicine development of new drugs.
(B) "medical preparations Registration" (Trial) provide that, under the theory of Chinese medicine prescription, prepared using traditional techniques (ie, the preparation process of preparation did not make the original group based prescription treatment of diseases of the material change), and The prescription of the medical institutions in more than 5 years (including 5 years) use of the history of Chinese medicine preparations, data entry can be free report 13-17.
Prepared using the traditional process is the preparation process is consistent with the traditional process, including the Chinese Herbal Medicine by crushing or water extraction is made solely by the solid, semi-solid and liquid dosage forms of traditional, modern formulations, but also the wine made by traditional methods agents, tincture.
The medical establishment has more than 5 years (including 5 years) use of history is in the medical institutions to provide more than 5 years of continuous use of text references (eg, prescription, record research projects, clinical swap records, etc.), and provide 100 cases over a relatively complete clinical records.
(C) of the medical institutions of traditional Chinese medicine preparation should focus on the clinical safety evaluation. Do not have the medical ethics committee set up to apply for clinical research of Chinese medicine preparations, the provisions could be entrusted to have been filed to the drug regulatory department and other medical institutions Ethics Committee for review.
(D) The following circumstances excluded from the scope of medical institutions, management of Chinese medicine preparations:
1. Chinese processed into powder, temporary use when adding water, wine, vinegar, honey, sesame oil and other Chinese traditional matrix deployment, external, in the medical institutions used by the deployment of medical personnel.
2. Fresh juice drug.
3. Commissioned by the patients, according to prescription (one side) application of traditional Chinese medicine processing of the products.
Fourth, improve the medical management of traditional Chinese medicine preparation in the preparation
(A) of the country should be promoting the "medical preparations prepared quality management practices" (for Trial Implementation) implementation, to strengthen the preparation of medical institutions, management of traditional Chinese medicine preparations, and constantly improve the quality of medical institutions, management of traditional Chinese medicine preparation.
(B) has been approved "hospital" category, traditional Chinese medicine preparation medical institutions, such as the preparation does not have the ability or lack of preparation conditions, the provincial food and drug administration approval, may appoint the area qualified medical or pharmaceutical preparation room preparation of manufacturing enterprises.
(C) "Chinese Pharmacopoeia" microbial agents not specified in General inspection requirements, and its agents do not require the preparation of clean area operations; non-sterile medicinal preparations net system, the rinse water and other pre-treatment and extraction can be used in accordance with standards of hygiene drinking water.
Fifth, to enhance the use of medical management of traditional Chinese medicine preparation
(A) of the medical institutions only in Chinese medicine preparations by the prescription of medical institutions, and is not on the market or through the Internet, mail order and other disguised sales, medical institutions shall publish the advertisements of Chinese medicine preparations.
(B) occurs disaster, epidemic, emergency or urgent clinical needs and the market is not supply, and other special circumstances, the State Council or provincial, autonomous regional and municipal people's government drug regulatory approval, preparations made by medical institutions in the designated medical transfers between institutions.
Meet the "medical organization product Registration" (Trial) Medical institutions to transfer the relevant provisions of national medicine preparations, the provincial food and drug administration approval, can be specified in this area Minzu Hospital Yiliao institutions and comprehensive medical hospital Minzu transfers between rooms, the specific implementation of the provisions of the provincial areas by the national drug regulatory department in conjunction with the medical management, combined with the actual situation in the region to develop.
(C) of the following circumstances of the medical institutions Traditional Chinese Medicine, Chinese Medicine by the provincial department of examination and approval, and by the provincial drug administration approval, specified in the administrative area of medical institutions to use. Subject to cross-jurisdictional use approval to the State Administration of Traditional Chinese Medicine, and the State Food and Drug Administration approval.
1. The Ministry of Health or the State Drug Administration approved the counterpart support.
2. National Key specialist technical collaboration.
3. National research project collaboration.
Application and approval, should provide relevant documents and identify quantity, intended purpose, scope and duration of use, the use shall not usually exceed 6 months.
Preparations obtained approval number should be approved for use in medical institution medical institutions responsible for the quality preparations. Medical institutions of preparations for preparation instructions should be strictly in accordance with, and beyond the scope of use or adverse effects caused by improper use responsibility.
Administrative departments at all levels of health, food and drug administration departments and the medical management of medical institutions to attach great importance to the development of Chinese medicine preparations, to further strengthen communication and cooperation, give full play the guiding role to ensure that the development of medical institutions in the direction of traditional Chinese medicine preparation and focus, and implement management of medical institutions of the provisions of traditional Chinese medicine preparation, strict, careful review, quality assurance, stress the characteristic, it is necessary to ensure Chinese medicine clinical drug safe and effective, but also give full consideration to hospitals and the actual needs of the people, and promote traditional Chinese medicine preparation medical institutions the healthy development and prosperity in the pharmaceutical industry.
Attachment:
Medical preparations Registration (for Trial Implementation)
State Food and Drug Administration Order No. 20
March 22, 2005 by the State Food and Drug Administration Bureau of Finance Council, is hereby promulgated, August 1, 2005 shall come into force.
June 22, 2005
Chapter I General Provisions
The first agents to strengthen the management of medical institutions, medical institutions standardize the reporting and approval of preparation, according to "Drug Administration Law of the People's Republic" (hereinafter referred to as the "Drug Administration Law") and "Implementation Regulations of the PRC Drug Administration Law" (hereinafter referred to as the "Drug Administration Law Implementing Regulations") regulations.
The second application of medical institutions in the PRC, the preparation of formulations, transfers, and the associated approval, inspection and supervision and management, application of this approach.
Article medical preparations, is the health care agencies, according to clinical needs of the unit is approved and preparation, personal use of the fixed-counter preparations.
Preparations made by medical institutions, should be no supply of varieties on the market.
Article State Food and Drug Administration is responsible for the supervision of the national medical organization product management.
Provinces, autonomous regions and municipalities (food) drug administration department is responsible for preparation of the area medical institutions for approval and supervision of management.
Article medical preparations applicants should be holders of "medical institution" and made "medical preparations permits" medical institutions.
Without a "medical preparations permit" or "medical preparations license" without the corresponding preparation formulation of the "Hospital" category of medical institutions, medical institutions can apply for traditional Chinese medicine preparation, but must also prepare their application for the commissioned. Entrusted with the preparation of the unit should be made "medical preparations permits" to obtain medical institutions or "Good Manufacturing Practice" certification of pharmaceutical production companies. Commissioned the preparation of formulations of preparations should be held with the trustees, "the medical organization product license" or "Good Manufacturing Practice" certification set forth in the same range.
Article medical preparations only in the medical institutions by practicing physicians or licensed assistant doctors prescription use, and with the "medical institution" set forth in the scope of the same treatment.
Chapter II Examination and Approval
Article VII application for medical preparations, should make the appropriate pre-clinical studies, including prescription screening, preparation process, quality indicators, pharmacology, toxicology studies.
To apply for registration by medical institutions to submit information on preparations should be true, complete and standardized.
Article IX of chemical raw materials used in application preparation and implementation of approval number of Chinese herbs, Chinese Herbal Medicine must have a drug approval number, and to meet statutory standards for pharmaceuticals.
Article applicant shall apply for registration of its agents or use of the prescription, process, use, etc., provide the applicant or others in the state of China's patent and ownership description; others in China has a patent, the applicant shall submit to others does not constitute an infringement of the patent statement.
Article XI The name of the medical organization product shall be in accordance with the State Food and Drug Administration promulgated the principles of drug naming names, trade names not be used.
Article XII of medical preparations, materials used and direct contact with agents of packaging materials, containers, etc., shall comply with the State Food and Drug Administration related materials, direct contact with packaging material and container regulations.
Article XIII medical preparation instructions and packaging labels provinces, autonomous regions and municipalities (food) drug administration department of the information submitted by the applicant, together with the approval of the application be approved agents.
Medical preparation instructions and packaging labels should be in accordance with the State Food and Drug Administration statement on pharmaceutical labeling and packaging regulations printed, its text, graphics shall not exceed the approved content and be labeled "The preparation is limited to the medical institutions "word.
Article XIV of the following circumstances, shall not be declared as a medical preparation:
(A) the supply of species already on the market;
(B) with the State Food and Drug Administration without the approval of the active ingredients of the species;
(C) In addition to allergens outside the biological products;
(D) traditional Chinese medicine injection;
(E) traditional Chinese medicine, chemical medicine preparation composed of the compound;
(F) of narcotic drugs, psychotropic substances, toxic drugs for medical use, radioactive drugs;
(G) does not conform to relevant regulations of other preparations.
Article XV preparation of medical preparations application, the applicant shall complete the "registration form medical preparations" to the provinces, autonomous regions and municipalities (food) drug administration authorities or the commission of the municipal districts (food) drug administration application, submit the relevant information and preparation of pure oxygen.
Article XVI of applications received by provinces, autonomous regions and municipalities (of food) drug administration authorities or the commission of the municipal districts (food) drug regulatory agency reporting data in the form of review to meet the requirements to be accepted; do not meet requirements, should be receiving the application materials within 5 days from the date of written notice of the applicant and explain the reasons for overdue notice from the date of receipt of materials shall be accepted.
Article XVII of provinces, autonomous regions and municipalities (food) drug administration authorities or the commission of the municipal districts (food) drug regulatory agencies should apply within 10 days after receiving the spot inspection, taking samples for testing for 3 consecutive batches, Drug specified in the notice for sample testing and quality standards technical review. Entrusted the municipal districts (food) drug regulatory agencies should work after the completion of the review comments, the information submitted to the inspection report and declaration of provinces, autonomous regions and municipalities (food) drug administration authorities, and notify the applicant.
Article XVIII received notice Drug testing should be completed within 40 days, sample test for technical review and quality standards, and standards inspection report issued review comments submitted to the provinces, autonomous regions and municipalities (food) drug regulatory department and copied to notice of its inspection of the (food) drug regulatory agencies and the applicant.
Article XIX of provinces, autonomous regions and municipalities (food) drug administration department shall within 40 days after receiving all the information organized and completed the technical review, compliance and issue a "clinical research medical preparations approval documents."
Applying chemical agents have been prepared to obtain agents with the approval of the text variety, and can be free from clinical research.
Diershitiao preparations used in clinical studies, it should be in accordance with the "preparation of the medical organization product quality management practices" or "Good Manufacturing Practice" requirements of preparation, medicinal preparations should conform to the provinces, autonomous regions and municipalities (food) drug administration authorities validation of the quality standards.
Twenty-one clinical study of medical preparations, should be "clinical research medical preparations this document" obtained after informed consent and subjects ethics committee approval, in accordance with the "drug clinical trial quality management practices" requirements of implementation.
Twenty-two medical institutions in preparation of clinical research, medical institutions should be in accordance with the clinical research program, the number of tested cases of not less than 60 cases.
Twenty-three clinical studies completed, the applicant to the local provinces, autonomous regions and municipalities (food) drug administration authorities or the commission of the municipal districts (food) drug administration institutions to submit the summary of clinical research data.
Twenty-four provinces, autonomous regions and municipalities (food) drug administration authorities to declare all the information received within 40 days after completion of technical review organization to make a decision whether to grant permission. Compliance, it shall make a decision to grant permission to the applicant within 10 days from the date of issuance of "medical preparations up this document" and the preparation approval number, while the State Food and Drug Administration for the record; non-compliance, should be written notify the applicant and state the reasons, and inform the applicant of the right to apply for administrative reconsideration or bring an administrative lawsuit.
Twenty-five medical institutions preparation approval number of the form:
X pharmacists word H (Z) +4 +4 bit serial number bit reign.
X-provinces, autonomous regions and municipalities referred to, H-chemical agents, Z-medicine preparations.
Chapter transfers
Twenty-six medical institutions generally not to transfer agents. Disaster occurred, the epidemic, emergency or urgent clinical needs and the market is not supplied, the necessary transfer, is a preparation of medical institutions within provincial jurisdiction swap, and must be approved by the provinces, autonomous regions and municipalities (food) drug regulatory authorities for approval; State Food and Drug Administration is under the special agents, and provinces, autonomous regions and municipalities directly under the swap between the preparation of medical institutions must be approved by the State Food and Drug Administration approval.
Twenty-seventh The provincial medical institutions within its jurisdiction to apply to transfer agents, it should be by the use of units to the provinces, autonomous regions and municipalities (food) drug administration departments to apply for, that use of reason, duration, number and scope, and submit relevant information.
Provinces, autonomous regions and municipalities directly under the swap between the use of medical preparations and the provisions of the State Food and Drug Administration to transfer the special agents, agents should obtain approval from the number of medical institutions to the provinces, autonomous regions and municipalities (food) drug management application, indicating that use of reason, duration, number and scope, the location of provinces, autonomous regions and municipalities (food) drug administration agreed to review, by the use of units will review the comments and relevant information shall be submitted to the use of units of the province , autonomous regions and municipalities (food) drug administration department and consent of the State Food and Drug Administration approval.
第二 eighteen preparations obtained approval of the text of the medical institutions should regulate the use of agents responsible for the quality of medical institutions. To accept transfers of medical institutions should be strictly in accordance with the manual preparation of preparations, and over the scope of use or improper use of the adverse consequences of responsibility.
Twenty-ninth a medical institution to transfer agents, shall not exceed the prescribed period, the number and scope.
Chapter IV Supplementary application and re-registration
The third ten medical preparations, should be strictly implemented by the approved quality standards, and technology may change, prescription, preparation of site and commissioned the preparation of units. Need to change, the applicant shall submit supplemental applications submitted to the relevant information, approved the rear executable.
第三十一条 medical organization product approval number is valid for 3 years. Expiry of the need to continue to compound, the applicant shall before the expiry of 3 months preparation procedures according to the original application for re-registration applications submitted to the relevant information.
The thirty provinces, autonomous regions and municipalities (food) drug administration department shall, after accepting an application for registration within 30 days and then make the decision whether to approve the re-registration. Granted re-registration, it shall make the decision to notify the applicant within 10 days, to renew, "medical preparations up this document", submitted to the State Food and Drug Administration for the record.
Decided not to re-registration shall notify the applicant in writing and explain the reason, and also advise the applicant of the right to apply for administrative reconsideration or bring an administrative lawsuit.
Thirty-third, one of the following circumstances, provinces, autonomous regions and municipalities (food) drug administration department will not approve re-registration and cancellation of preparation of approval:
(A) the supply of species already on the market;
(B) Measures should be removed in accordance with the number of approval:
(C) is not within the specified time the re-registration application;
(D) other non-compliance.
Thirty-fourth Ordinance already been written off approval number of medical institutions preparations, no preparation and use; has been prepared by the local (food) drug administration authorities supervise the destruction or treatment.
Supervision and Administration Chapter
第三 fifteen formulation and use of medical preparations to observe the preparations should be adverse reactions, and the State Food and Drug Administration in accordance with the relevant provisions of reporting and processing.
Thirty-sixth of the provinces, autonomous regions and municipalities (food) drug administration department of the unstable quality, efficacy inaccurate, adverse reactions, or other harmful agents to human health care institutions, medical institutions should be ordered to stop the preparation, and to withdraw its approval symbol.
No approval has been revoked the medical organization product, not the preparation and use; been prepared by the local (food) drug administration authorities supervise the destruction or treatment.
A medical institution thirty-seventh random testing agents, in accordance with the State Food and Drug Administration drug sample test relevant regulations.
Thirty-eighth preparation of a medical institution no longer has the qualifications or conditions, formulations, and its agents to obtain the corresponding approval number shall prevail, and the provinces, autonomous regions and municipalities (food) drug administration authorities to be canceled, but the commission prepared to allow except for traditional Chinese medicine preparation approval number. Commissioned the preparation of traditional Chinese medicine preparation to allow for continued preparation, can refer to this approach the preparation of the third unit of ten changes to the provisions of commissioned commissioned the preparation of the supplementary application.
Thirty-ninth without approval, medical institutions, medical institutions unauthorized use of other formulations prepared in accordance with "Drug Administration Law" Article ten penalty shall be imposed.
Preparations, the fourth ten medical institutions, in violation of "Drug Administration Law" fourth eighteen, the provisions of Article respectively under the "Drug Administration Law" seventy-fourth and Article fifteen of the penalty shall be imposed.
Fails to provinces, autonomous regions and municipalities (food) drug administration approval of the standard preparation of formulations, a "Drug Administration Law," third paragraph of Article VI of the forty-ninth of other drugs do not meet the standard requirements of the case, according to the "Drug Administration Law "seventy-fifth Article shall be punished.
第四十一条 providing false documents, claims data, samples, or by other fraudulent means approval documents, the provinces, autonomous regions and municipalities (food) drug administration departments of the application as inadmissible, the applicant be given a warning, the application will not be accepted within one year; approval documents has been made to withdraw its approval documents, the application will not be accepted within five years, impose a fine of thirty thousand yuan yuan.
Forty-second preparations made a medical institution shall not be disguised in the market or sell, not release medical preparations ads.
Medical institutions of their preparations on the market or a disguised sales, according to "Drug Administration Law" eighty-four penalty shall be imposed.
Forty provinces, autonomous regions and municipalities (food) drug regulatory department of administrative acts in violation of the Measures, the State Food and Drug Administration should order them within a time limit; overdue correction by the State Food and Drug Administration to be changed or revoked.
Chapter VI Supplementary Provisions
Provisions of the forty-fourth article this way the executive branch to implement the administrative licensing time working days, excluding legal holidays.
Forty-fifth of this approach in the "fixed counter preparations" means preparations fixed prescription, preparation of process maturity, and long-term use in the clinical preparation of a particular disease.
Forty-sixth of the provinces, autonomous regions and municipalities (food) drug administration department under this approach, combined with the practical details of implementation of local.
Forty-seventh of this approach since August 1, 2005 shall come into force.