中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
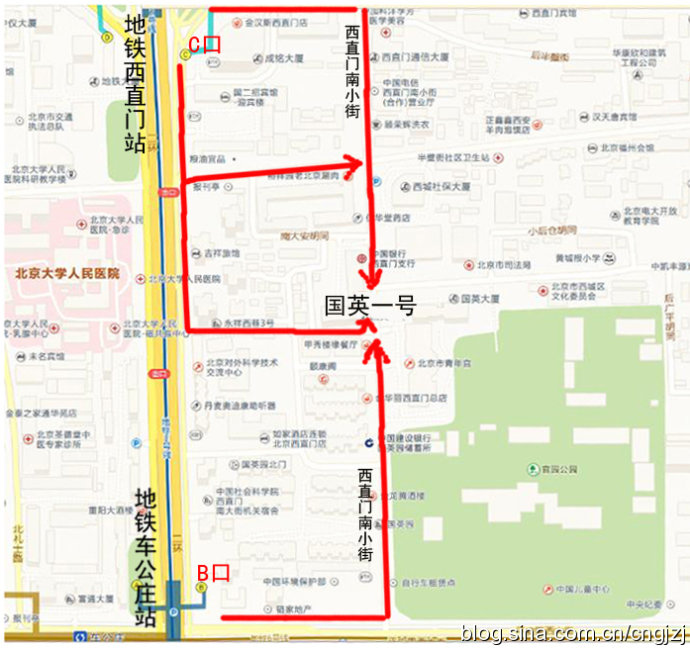
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
2016年05月17日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Generics China can learn from India?
May 17, 2016
Source: southern metropolis daily
Author: NieRi Ming
Recently, some events to detonate public opinion, India's production of generic drugs to enter the public discussion. Have a kind of medicine, the dose of a month, selling more than 40000 yuan in Hong Kong, China, in India only 5000 yuan, and curative effect.
India is "world pharmacy". This is because the Indian patent law to allow drug compulsory license, when "the public is the patent of invention reasonable unmet demand", or "the public can't at a reasonable price paid to obtain the patent invention", and so on and so forth, Indian pharmaceutical factory in local can forcibly generic drugs is under patent protection, and can be exported to no relevant production capacity of regions and countries. China's patent law framework, unlike India, as a world power, also have a duty to maintain the intellectual property rights, can't learn from India's generic drug patent protection.
But India can become a "world pharmacy", is not only the characteristics of the patent law, the quality of Indian generics, almost with the original drug.
The original drug research and development is complex, a new drug to first know during development components (generally refers to the formula), clear mechanism, pharmacology and toxicology, also need to pass strict animal experiment and human clinical one, two, three phase double blind test, and then amplified by phase iv clinical experiment proves curative effect is accurate, safe and reliable to the marketing.
Generic drugs refers to when the original drug patents expire, other pharmaceutical factory imitation branded drugs. Generic drugs generally do not need to bother, just to prove their generic medicines in dosage, safety and efficacy, and quality, effects and indications of factors are consistent with the original drug, can sell, its good or bad usually by generics and branded drug to judge the consistency of the evaluation, namely "bioequivalence test".
India is a generic power, produces 20% of the world's generic drugs, India's pharmaceutical exports to more than 200 countries, vaccines and biological pharmaceutical products are exported to 150 countries. Media reports, India's generic drugs more than 60% of exports to the us and Europe, and other developed countries, including the United States the generic drugs on the market nearly 40% from India.
Indian generics can open the us market doors in addition to the low price factors, mainly because the quality is reliable. Besides consistency evaluation, India's best pharmaceutical factory production management specification directly follow the FDA (food and drug administration) certification, is currently in India with FD A certified pharmaceutical factory 119, with the drug administration approved pharmaceutical factory has more than 80.
The generic word of mouth is just the opposite in China. Media reports, to be on the safe side, the domestic some well-known hospital intensive medication and other special departments, general advice to choose imported drugs. General common medicine (e.g., antibiotics), domestic generics allergic reaction, and the efficacy is not exact, to try again on the underlying original drugs, is no problem.
Why is this so? Earlier generic drug market in China, regulators in order to promote the development of generic drugs, generic consistency evaluation does not need to directly to the original drugs, generic drugs may also be listed. The personage inside course of study points out, "to the" consistency of the subject matter of the expanded from generics not like "the imitation of the", such as A generic drugs and the consistency of the original drug evaluation within 10% can be listed, B of generic drugs and A 10% difference can also be listed again, A few rounds of accumulated, generics and branded drug efficacy had no consistency.
China's drug manufacturers, technology, process, quality control key links such as is not satisfactory. Drug regulatory agencies in the early 1990 s began to promote pharmaceutical production specification operation, carry out drug GMP certification (GoodM anufacturingPrac - tice, excellent manufacturing standards), but the GMP standard and American FDA certification standards far, execution strength is not strong. Successively in 2006 "effectively" and "xin" event is the cause of drug production operation process is not in conformity with the specification, the drug companies are involved by G M P certification enterprises.
The consistency of Chinese generic drug evaluation is not standard and quality control standard of backward, seriously affected the curative effect of generic drugs. Viagra patent expires in 2014, the domestic drug firms began to copy, but the effect is poor, which makes the original drug viagra still by domestic patients, drug prices did not decline on schedule.
The curative effect of Chinese generic drug to agree with the original drug, it is necessary to learn from India's drug companies, on the basis of complete consistency evaluation, comprehensive production quality control to the FD. A certification of standard. Since 2014, the quality control of drug regulatory agencies to promote strong generics, start a new G M P certification, also requires that all manufacturers to the original medicine as "the standard", carry out the consistency evaluation, correct historical error, it reverses the most generics companies existing production standards and procedures. This is a good news, if done right, can change the current strong generics market turmoil.