中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
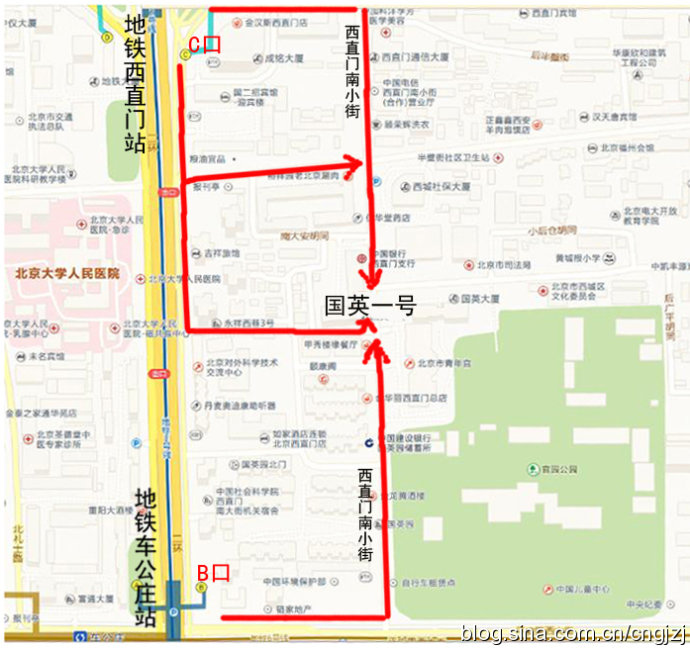
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
2016年11月09日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Human medicinal center data extrapolation power children medicine research and development are put forward
On November 7, 2016
China pharmaceutical net
At present, China pharmaceutical industry dynamic 】 【 children medicine is faced with "no medical pediatrics", "adult medicine break half" awkward situation. Now, drug firms likely to use the data extrapolation to perfect and enrich pediatric population information.
Released on November 3, the adult dosage data extrapolation in the pediatric population of drug clinical trials and the relevant information of the technical guidelines "(draft), the human medicinal drug safety administration center (CDE) is put forward," as far as possible, reduce the pediatric population the number of subjects in drug clinical trials, through data extrapolation to improve drug pediatric population and rich information, to guide the clinical medication, become the important and necessary way of ensure the safety of children with drug use."
CDE also said that guidelines with emphasis on the concept and framework of the data extrapolation, pediatric population drug clinical trial data extrapolating the basic principles and requirements, provide the reference for the research and development enterprise.
"Considering the target group, the differences between the treatment and drug properties, data extrapolation method is its diversity and flexibility, this guiding principle only data extrapolation general considerations are put forward."
"Encourage the applicant in the pediatric population in the early stages of drug development and regulatory data extrapolation to communicate."
Research and development institutions should also reference drugs quality control standard for clinical trials (GCP), people with drug registration specification international coordination meeting (ICH) and other relevant guidelines require both at home and abroad, the CDE said.
This guideline is only on behalf of the drug regulatory agency of the current views and understanding, with the progress of the scientific research related content in this guideline will constantly improve, human medicinal center added.
Solicit opinions from the date of release as of two months. Can be in CDE fill send directly on site. (source: the healthy idea ihealth)