中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
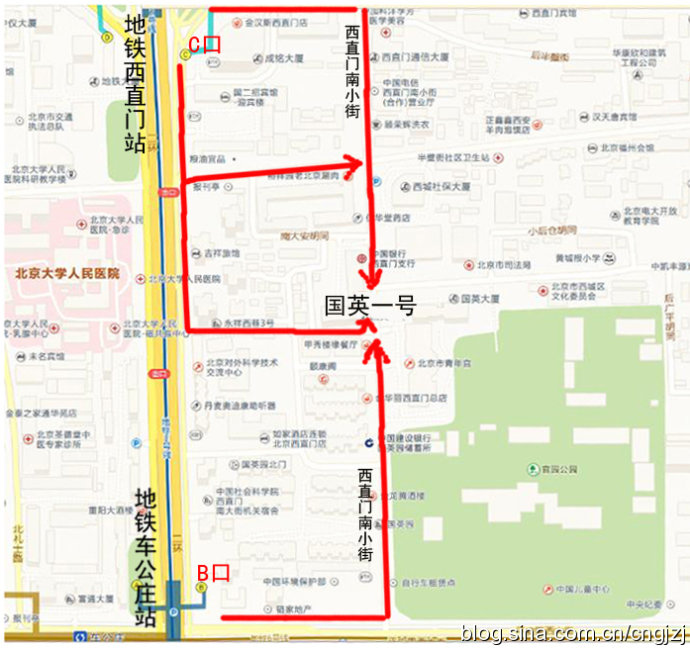
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2018年04月26日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
The state drug administration bureau promulgated the technical guiding principles for the revision of the processing specifications of the provincial traditional Chinese medicine
Enhance the quality controllability of Chinese herbal medicine
Time: 2018-04-23 source: Chinese traditional Chinese medicine report 1 edition author: chestnut sign
Report from our correspondent (reporter chestnut) in order to strengthen the management of the Chinese medicine yinpian, standardize the provincial Chinese medicine yinpian process for revision of the work, enhance the quality of Chinese medicine yinpian controllability, the state drug administration (fda) has issued the provincial Chinese medicine yinpian process for revision of the technical guiding principles, points out that the local pharmaceutical supervisory and administrative departments should be organized set up provincial yinpian processing standard revision work committee, responsible for the design and technical review.
"Guiding principles", stressed that the provincial yinpian processing should insist on the guidance of TCM theory, adhere to the revision of the norm in accordance with the standard revision, insist on the inheritance and protection of local characteristics, study the scientific and rigour, adhere to scientific and technological innovation and development.
The guidelines set out six basic requirements. One is revised provincial yinpian processing specification, deal with slices of medicinal plant, animal, mineral) varieties, character, origin, resources situation, the origin of processing methods, processing history, processing technology and its research progress, and quality control, clinical application and so on to conduct a comprehensive investigation and research; In addition to the requirements of the normal slices, the effect of the processing technology on the safety of the tablets should be investigated.
Second, the provincial yinpian processing specification should be in strict accordance with the relevant provisions of the pharmaceutical administration law and its implementing regulations, it contains only with local feature and history of clinic conventional varieties; It is not allowed to collect the scientific research products that are not in the scientific research stage without the recognized safety and effectiveness data, as well as the products controlled by tablets and granules, etc.; It should not be accepted as a specification for the consumption of the powder, except for the actual history of clinical practice.
Third, the content of the processing standard of the provincial drinking chip should be made according to the relevant requirements of the province and the quality control. The layout and body parts can be generally referred to the standard items and formats of the drinks in the current Chinese pharmacopoeia.
Fourth, the writing specification for the processing standard of provincial drinking chip can be carried out in reference to the current version of the technical specification of national drug standards. The terms, symbols, units of measurement, general codes, inspection methods and related requirements shall be carried out in accordance with the provisions of the Chinese pharmacopoeia.
5. All sample information, original records, pictures and other documents, samples and samples should be kept for archival purposes.
Six is released to the country "prohibited export restrictions on technology export management method" and the catalog of varieties of national science and technology secret contains yinpian processing technology or key varieties, shall comply with the relevant state confidentiality system, and the key processing technology and technological parameters in the release of the specification shall be reserved.
"Guiding principles", according to local pharmaceutical supervisory and administrative departments should be organized yinpian processing production, within their respective administrative areas, clinical application, analysis and inspection, drug supervision, and relevant experts, set up provincial yinpian processing standard revision work committee, responsible for provincial yinpian process for the project design and technical audit work. In general, the revision of the processing specifications of provincial slices should be carried out in an orderly manner according to the selection of varieties, collection of samples, research and development, technical review, consultation and release.
The guiding principles also put forward the requirements for the research work and technical examination of the processing standard of the provincial drinking chip. This guideline can be referred to in the revision of the processing standard of ethnic minority medicines.