中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
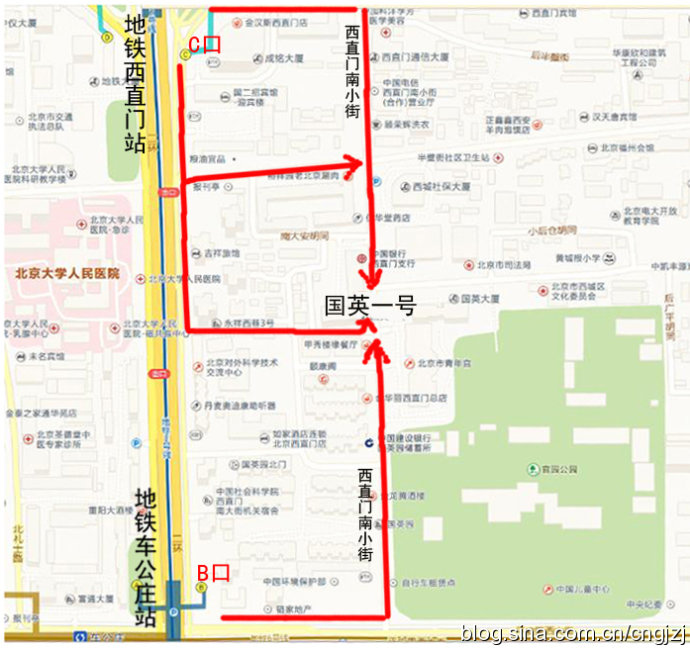
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
您现在的位置是:
首页 >>
R&Dplatform >>
R&Dplatform
2018年05月10日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Technology daily reporter sun yu-song reporter wu junhui.
Today, reporters learned from nankai university, the school element state key laboratory of organic chemistry professor Chen bow group have developed a powerful methods of synthesis of cyclic peptide compounds that plagued chemical industry for many years, "difficult polypeptide cyclization reaction" to realize the efficient and controllable. As an important breakthrough in the synthesis of cyclic peptides, the research also provides a novel design "tool" for the development of peptides. The results are published in the latest issue of the international journal nature chemistry.
According to introducing, compared with the traditional small molecule compounds, by a variety of amino acid unit in series of peptides compounds in building a larger molecular structure have unique advantages and potential, to known of cyclic peptide compounds including anti-tumor, anti-hiv, antibacterial, malaria, sleeping, inhibit platelet aggregation, immunosuppression and other aspects of biological activity. Both studies show that let the polypeptide chain "ring" can be in structural robustness, across cell membrane, metabolic stability significantly improved many ways, such as peptides into medicinal properties, and although in the past few decades of cyclic peptide synthesis chemistry has made considerable development, but still has great limitations, how to make these "big" "shape" for the ideal of peptide three-dimensional structure (cyclic peptide) and has good pharmacological properties and the great challenge.
Inspired by cyclic peptide the biosynthesis of natural products, Chen bow team of chain through metal catalytic peptides on the substrate was very inert alkyl hydrocarbon selective activation key, and with iodine and replace the aromatic amino acid side chains in intramolecular coupling to generate a variety of ring products. This method the palladium catalytic alkyl hydrocarbon activation key cleverly used in the synthesis of complex polypeptide, to "tame" many hard cyclization of linear peptide precursor, let them n-cyclohexylmaleimide "darling". Because the method USES the unconventional hydrocarbon key activation "synthetic strategy, method is simple and efficient, the substrate scope is wide, not only overcome the long plagued" peptide cyclization reaction substrate dependence, but also the efficient preparation has a unique "benzene racks" skeleton structure of three-dimensional cyclic peptide, to build different volume of cyclic peptide compounds provides a new universal way, also to find more good pilot drug activity, new type of cyclic peptide compounds promote targeting peptide drug development laid a solid foundation.