中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
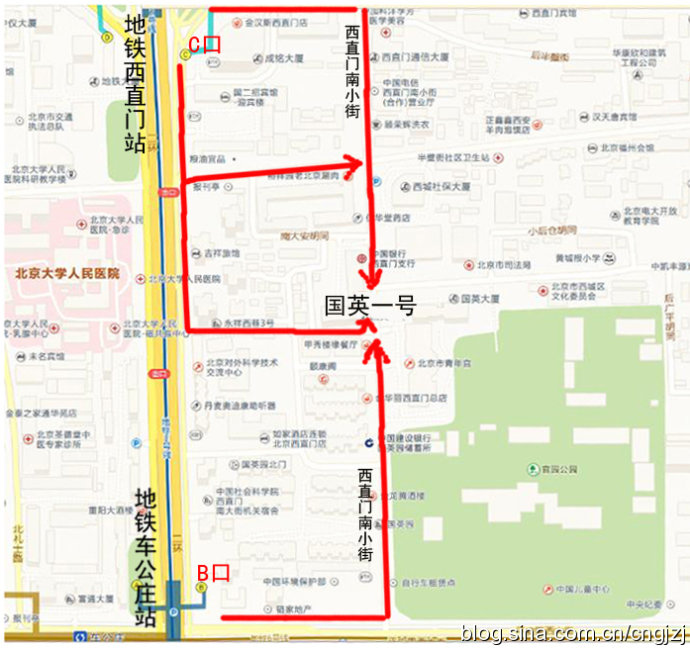
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2018年09月20日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
The general office of the state council has issued the opinions on perfecting the national basic drug system.
In 2018-09-20, the official xinhua news agency
The general office of the state council has issued the opinions on improving the country's basic drug system (hereinafter referred to as the opinions), xinhua reported.
The opinions proposed that since the new round of medical reform, the establishment and implementation of the national basic drug system has played an important role in improving the drug supply and security system, ensuring basic drug use by the public and reducing the burden of medication on patients. At the same time, there are still some problems, such as incomplete adaptation to the needs of basic clinical medication, lack of incentive mechanism for use, gap in quality and efficacy between generic varieties and original research varieties, and imperfect supply guarantee mechanism.
"Opinions" pointed out that to fully implement the party's 19 large and the 19th session of 2, the third plenary session of the spirit, the thought of socialism with Chinese characteristics in jinping new era as a guide, adhere to people's health as the center, strengthening basic drugs "outstanding required, ensuring the supply of basic, prevention and cure, use priority, ensure the quality, to reduce the burden" of function orientation, comprehensively promote the drug supply security system construction, vigorously ensuring drug is safe and effective, reasonable price, adequate supply, to alleviate "see a doctor expensive" problem. We will promote the linkage of drug use between higher and lower medical institutions, facilitate the development of a hierarchical medical treatment system, and promote the transformation and upgrading of the pharmaceutical industry and supply-side structural reform.
From the selection, production, circulation, use, payment and monitoring of basic drugs, the opinion defines five aspects of policies and measures. One is to dynamically adjust and optimize the directory. Regular evaluation and dynamic adjustment of the catalog of essential drugs, highlighting the clinical value of drugs, adhering to the equal emphasis on Chinese and western medicines, meeting the major clinical needs of common diseases, chronic diseases, emergency rescue, and giving consideration to the needs of special groups such as children and public health prevention and treatment. Second, we will effectively guarantee production and supply. We will adhere to the direction of centralized procurement and implement classified procurement of drugs. We will improve the connection between medical institutions at the upper and lower levels, promote centralized procurement of public medical institutions within the city (county) area, and drive down drug prices. To be easy to shortage of basic drugs, through market matching to determine reasonable purchase price, designated production, uniform distribution or included in the reserve measures to ensure supply. Third, it is fully equipped for priority use. We should adhere to the leading role of essential drugs and clarify the proportion of essential drugs in public medical institutions. Clinical use monitoring and comprehensive evaluation of drugs were carried out. We will deepen reform of the payment method for medical insurance, formulate payment standards for medical insurance, and guide rational diagnosis and treatment and rational use of medicines. Fourth, we will reduce the burden of drug charges for the general public. Priority should be given to including basic drugs in the list of medical insurance, and the level of practical support should be gradually improved. Local authorities are encouraged to explore effective ways to reduce patient burden and minimize patient drug expenses. Fifth, improve the quality and safety level. We will carry out spot checks for all kinds of essential drugs, strengthen supervision and inspection of the production of basic drugs, and strengthen quality and safety supervision. For the drug varieties that have passed the consistency evaluation, the list of basic drugs should be included in priority according to the procedure. The essential drug varieties that have not passed the consistency evaluation are gradually brought out of the list.
The guideline calls for strengthening organizational leadership and governments at all levels to incorporate the implementation of the national basic drug system into the government performance appraisal system. We should strengthen the supervision and evaluation, establish and improve the basic drug system, implement the supervision and evaluation system, give full play to the role of third-party evaluation and strengthen the application of results. We should strengthen publicity and guidance, strengthen policy interpretation, and create a good social atmosphere for the implementation of the basic drug system.