中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
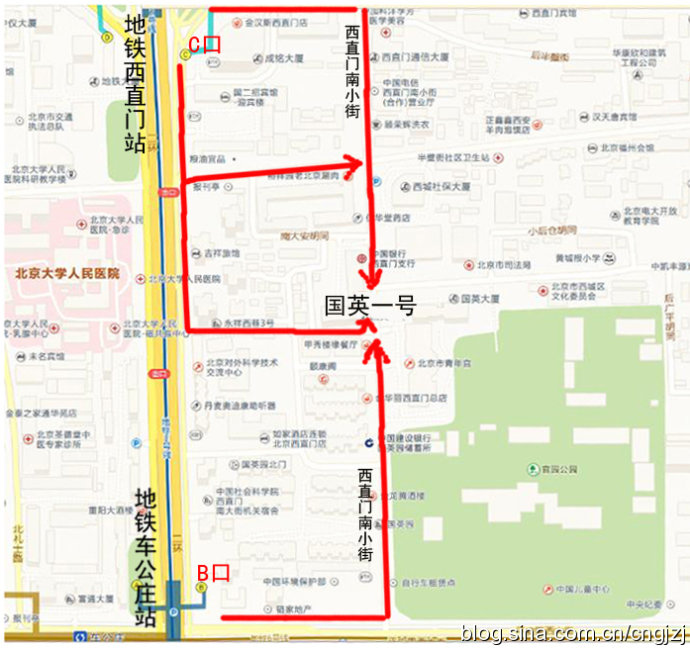
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2018年10月08日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
State council revises regulations on the protection of traditional Chinese medicine varieties
The drug administration department is responsible for the protection and supervision of traditional Chinese medicine varieties
Time: 2018-10-08
Recently, promulgated by the state council on amending part of the decision of the administrative regulations, the regulations on the protection of TCM variety made several changes, the original more provisions in the regulations of "the health administration department under the state council" changed to "the pharmaceutical supervisory and administrative department under the state council", explicitly by the pharmaceutical supervisory and administrative department under the state council is responsible for the supervision and administration of the national protection of TCM variety.
This modification has been cancelled to apply for in the regulations of the protection of TCM variety of programs to be transferred as prescribed by the administrative department of health, at the same level changed to "to the local people's governments of provinces, autonomous regions and municipalities directly under the central government pharmaceutical supervisory and administrative department, by the people's governments of provinces, autonomous regions and municipalities directly under the central government pharmaceutical supervisory and administrative department of the first trial after signing, reported to the pharmaceutical supervisory and administrative department under the state council. Under special circumstances, traditional Chinese medicine manufacturers may also apply directly to the drug regulatory authority under the state council. At the same time, the responsibility of entrusting the review committee for the protection of traditional Chinese medicine varieties, organizing the review committee for the protection of traditional Chinese medicine varieties, and employing experts of traditional Chinese medicine as members of the committee will be entrusted to the drug regulatory department under the state council.
In addition, the regulations on the imitation of protected varieties of traditional Chinese medicine have been adjusted. Article 19 of the regulations is amended to read: "the imitation of protected varieties of traditional Chinese medicines in shortage of clinical medication shall be approved by the drug regulatory authority under the state council and the approval document number shall be issued. The imitation enterprise shall pay reasonable royalties to the enterprise that holds the certificate of protected variety of traditional Chinese medicine and transfers the prescription composition and process method of the traditional Chinese medicine variety, the amount of which shall be agreed by both parties. If the parties fail to reach an agreement, the drug regulatory authority under the state council shall make a ruling.