中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
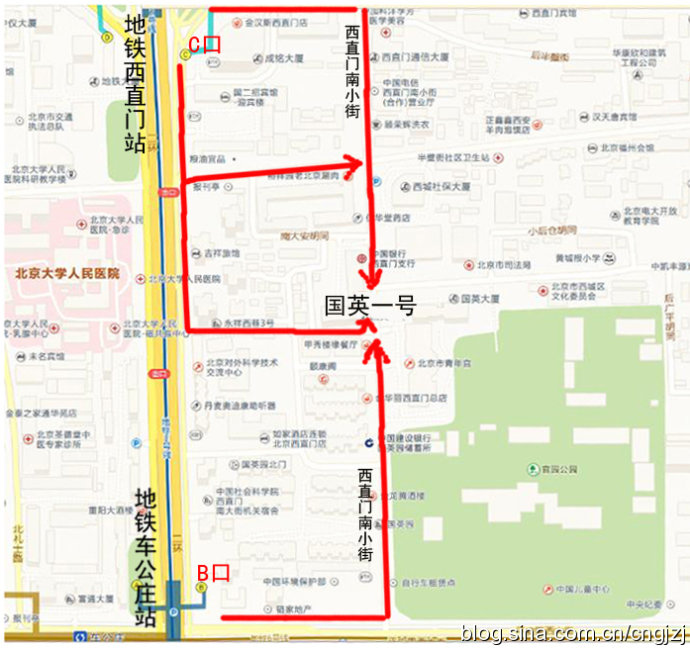
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2018年11月19日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Improve the quality of traditional Chinese medicine by scientific modification
The 2018-11-09 health
At present, the traditional Chinese medicine industry has become a strategic industry of China's national economy. With the steady progress of the modernization and internationalization of traditional Chinese medicine, it will surely play a greater role in the major strategies such as serving "One Belt And One Road". The premise of all this is to have safe, effective and controllable quality of Chinese medicine products. If the quality of traditional Chinese medicine products as the "fruit", its "cause" and what?
In the author's opinion, there are no more than the following two: one is predestined raw material quality. The quality of Chinese medicinal materials originates from nature and is affected by various factors, such as variety basis source, producing area (climate, soil), growth year, planting (breeding) way, harvesting season and processing method. Although many scholars have conducted in-depth studies from the aspects of source identification, scientific planting and evaluation standards, the quality of Chinese medicinal materials is too complicated to find scientific intervention methods, making the quality of raw materials consistent. Second, technological factors in the production of traditional Chinese medicine products. For example, decoction, percolation, concentration, alcohol precipitation, chromatography, extraction, mixture, pressure and other processes depend on a variety of process parameters. For a long time, people used to produce according to the established process parameters, but the question came: can the variable raw materials, together with the fixed process, get the consistent product? The answer, of course, is no.
Quality control to avoid "qualified fake drugs"
How can we find a way to manufacture traditional Chinese medicine products? In my opinion, we should start from the following two aspects:
First, the quality of raw materials should be controlled. No matter how complicated it is, no matter how expensive it is, no matter how expensive it is. If the raw material is inferior in quality, no matter how it is processed, the quality of the final product will be changed. Therefore, the quality control of raw materials must be implemented. The question is how do we control it. For a long time, both in the policy level, and the actual practice, people used to place the individual chemical composition content as the golden rule to control the quality of medicinal materials, it is, of course, policies and regulations of the enforceability, standardization of the operation factors, but also increasingly cause the question of practitioners, set standards for medicine, for example, high levels of measurement, what easy to test what, what what standard measurement... However, there is no in-depth study on the relationship between these indicators and safety and effectiveness, and insufficient attention is paid to the synergistic effect between the chemical components of traditional Chinese medicine, so that "qualified fake drugs" eventually appear.
Of course, Chinese scientists are aware of the shortcomings of current methods and are working to improve them. For example, the concept of "quality markers of traditional Chinese medicine" put forward by liu changxiao, an academician of the Chinese academy of engineering, and the concept of "biological evaluation of traditional Chinese medicine" put forward by professor xiao xiaohe, director of the whole army institute of traditional Chinese medicine of the PLA 302 hospital in recent years are all very beneficial attempts.
The author thinks that, in the implementation of Chinese medicinal materials quality control, must consider the overall properties of kinds of material (such as flavonoids, phenolic acids, saponins) total amount and composition, small molecules and macromolecular substance, water-soluble ingredients and fat-soluble composition proportion, how the interactions between the various ingredients, etc. Therefore, it is necessary to explore new analytical methods besides chromatography, which can reflect the whole nature of raw materials more comprehensively and comprehensively, and set a quantifiable control range for it, so as to achieve the real quality control.
Second, in the process of traditional Chinese medicine production, we should carry out the concept of flexible technology. The so-called flexible process is to find the best matching process and parameters according to the quality fluctuation of raw materials, so as to obtain the Chinese medicine products with high consistency. This is essentially the feedforward control concept applied in the field of traditional Chinese medicine production, in the understanding of material properties, process parameters and product quality on the basis of the relationship between process parameters optimization model is established, and then according to the material properties to calculate the appropriate process parameters scope of operation, thereby reducing the raw material properties wave effect on the product quality. This concept is conducive to improving the quality of traditional Chinese medicine products and the robustness of the production process, which is of great significance for the standardization and modernization of traditional Chinese medicine production.
How to change the production process of traditional Chinese medicine
Happily, the former state food and drug administration has been aware of the deficiencies in the original process of traditional Chinese medicine production, and has organized and formulated the technical guidelines for the study on the change of production process of listed traditional Chinese medicine, which has pointed out the direction to solve the current dilemma of traditional Chinese medicine production. The author studied this legal document carefully, among which the following highlights are:
Firstly, the process change is divided into three categories: minor change, moderate change and major change. The purpose of the classification is to facilitate the applicant to determine the content of the change research and to carry out the research effectively. However, no type of change should negatively affect the safety and efficacy of drugs. This regulation can also be interpreted as, as long as there is no negative impact on the safety and effectiveness of drugs, process changes can be considered. Of course, process changes should be able to stand examination, and the effect should be able to stand examination.
Second, the study on the technological change of listed traditional Chinese medicine should follow the principle of "necessary, scientific and reasonable" and the principle of "safe, effective and quality control". The process change is proposed and studied based on the understanding of the proposed process, based on the previous process research stage and the research and data accumulation in the actual production process. The more systematic and in-depth the research work in the early stage, the more abundant the data accumulated in the production process, and the more helpful it will be for the research on process change after going on the market. The applicant shall have a comprehensive and accurate understanding of the product development and production process, the nature of the product, etc., and shall have a clear understanding of the cause and degree of the change, and carry out the research on production process change with the idea that "quality comes from design".
Thirdly, in the submitted process change declaration data, it is required to briefly describe the selection basis and optimization process of the production process, list the corresponding process steps and the control range of main process parameters, and provide detailed process route, process method, process parameter definition, or research data of the type and dosage screening of auxiliary materials. In the author's opinion, "main process parameter control range" has rich connotation, first of all, it is the main process parameter, not all process parameters. The second is the control range, not the fixed process parameters. In this way, the modified process can be fine-tuned within the allowable range to adapt to the change of raw materials.
Although the guidelines have been ridiculed on the Internet by some practitioners, it will undoubtedly play a significant role in promoting the intelligent manufacturing of Chinese traditional medicine, improving the traditional production mode and production process for historical reasons, and comprehensively improving the production level and quality of Chinese traditional medicine products.