中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
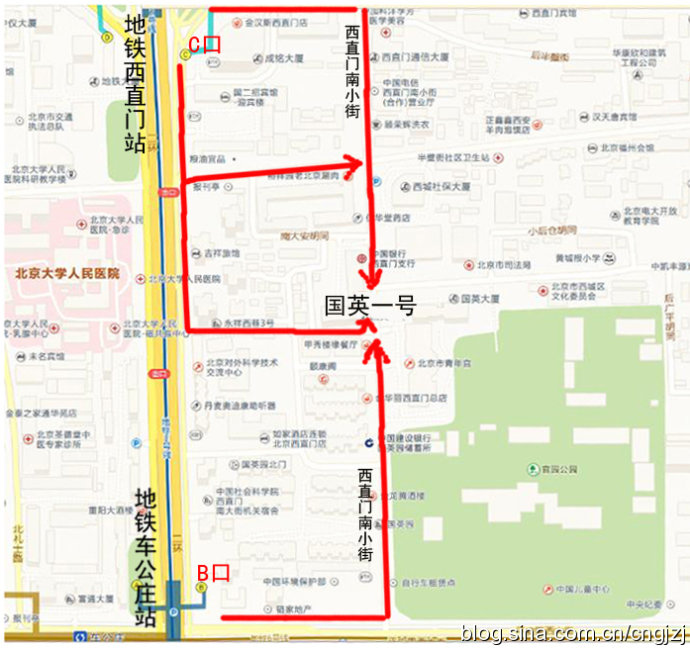
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2018年12月30日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Interpretation of Chinese Medicine Law(50)
Article 50 The State shall strengthen the system of standards for traditional Chinese medicine, formulate standards for the technical requirements that need to be unified according to the characteristics of traditional Chinese medicine, and revise them in a timely manner.
The national and trade standards for Chinese medicine shall be formulated or revised by the relevant departments under the State Council in accordance with their duties, and published on their websites for free public consultation.
The state promotes the establishment of an international standard system for Chinese medicine.
This article is about strengthening the construction of the standard system of traditional Chinese medicine.
Standards are unified technical requirements for products, services, etc.. They are the technical support for economic activities and social development. Strengthening standardization and improving the standards system will help promote technological progress, improve the quality of products and services, and modernize national governance. Standardization of TCM is the technical support for the development of TCM and the basic system for modernizing the governance system and capacity of TCM industry. The Outline of the Strategic Plan for the Development of Traditional Chinese Medicine(2016-2030) proposes: "We will improve the system of standards for traditional Chinese medicine. In order to ensure the safety of the quality of TCM services and implement the TCM standardization project, we will focus on the formulation, promotion and application of guidelines for clinical diagnosis and treatment of TCM, technical operation standards, and evaluation standards for its efficacy. We will systematically carry out research and formulation of standards for the treatment of diseases in China, standards for the production of medicinal meals, and standards for traditional Chinese medicine health products.
We will improve the quality standard system for traditional Chinese medicines, strengthen the quality management of traditional Chinese medicines, and strengthen the standard formulation and quality management of traditional Chinese medicines, traditional Chinese medicine identification, traditional Chinese medicine preparations, traditional Chinese medicine formula particles, and traditional Chinese medicines. Accelerate the construction of digital standard of traditional Chinese medicine and specimen of Chinese herbal medicine. Strengthening the system of standards for traditional Chinese medicine should conform to the characteristics of traditional Chinese medicine and follow the rules of traditional Chinese medicine. There is no need to formulate standards for standardized management of Chinese medicine services and products that do not require or are not suitable for formulating unified technical requirements. Otherwise, it will not only play a standardized role. On the contrary, it will limit the development of Chinese medicine characteristics and hinder the development of Chinese medicine. In determining which areas require uniform standards, the important feature of TCM syndrome differentiation and treatment must be taken into account.
In addition, the relevant departments should follow up and evaluate the effectiveness of the standards for traditional Chinese medicine that have already been formulated, so as to obtain information on the implementation of the relevant standards. Collect the scientific, rational and practical information of the relevant standards, indicators and technical requirements, and feedback from relevant enterprises. The standards shall be evaluated on the basis of the information collected and the problems identified shall be revised in a timely manner.
Chinese medicine standards cover a wide range of Chinese medicine services and products such as Chinese medicine medical services, Chinese medicine health care services, Chinese medicine, and Chinese medicine medical devices. Article 6 of the Standardization Law stipulates that national standards shall be formulated for technical requirements that need to be unified nationwide. National standards shall be formulated by the administrative department for standardization under the State Council. Industry standards may be formulated for technical requirements that do not have national standards and need to be unified within a certain industry nationwide. The industry standards shall be formulated by the relevant administrative department under the State Council and reported to the administrative department for standardization under the State Council for the record. Where the law provides otherwise in respect of the formulation of standards, the provisions of the law shall prevail.
Article 32 of the Law on Drug Administration stipulates that the Pharmacopoeia of the People's Republic of China and the drug standards promulgated by the drug regulatory department under the State Council shall be the National Drug standards. Article 44 of this Law stipulates that the norms and standards for health care services for traditional Chinese medicine shall be formulated by the competent Department of Chinese medicine under the State Council. According to the above provisions, the National standards and trade standards for Chinese medicine in different fields shall be formulated by the administrative department for standardization under the State Council, the drug regulatory department under the State Council, and the competent department for Chinese medicine under the State Council.
With regard to the functions and responsibilities for standard-setting, this article only provides for cohesion, and the specific standard-setting departments shall be determined in accordance with the provisions of the relevant laws. The content of the standard shall be published in its entirety on the website of the standard-setting department and made available to the public free of charge, irrespective of which department has formulated the national and industry standards for Chinese medicine and whether the standards are mandatory or recommended. This will make it easier for the public to be informed of the content of the standards in a timely manner, to carry out activities in accordance with the standards and to effectively play the role of the standards.
Standards play an important role in international trade. The establishment of an international standard system for Chinese medicine is conducive to promoting the international dissemination and application of Chinese medicine and promoting the "going global" of Chinese medicine. In September 2009, under the proposal of our country, the International Organization for Standardization established a special technical committee on Chinese medicine. At present, it has issued international standards such as "The one-time use of sterile acupuncture needles". China will continue to deepen exchanges and cooperation with the International Organization for Standardization(ISO), actively participate in the study and formulation of international standards for TCM, and create an international environment conducive to the development of TCM overseas. (From "Interpretation of the Chinese Medicine Law of the People's Republic of China", China Democratic Law Publishing House)