中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
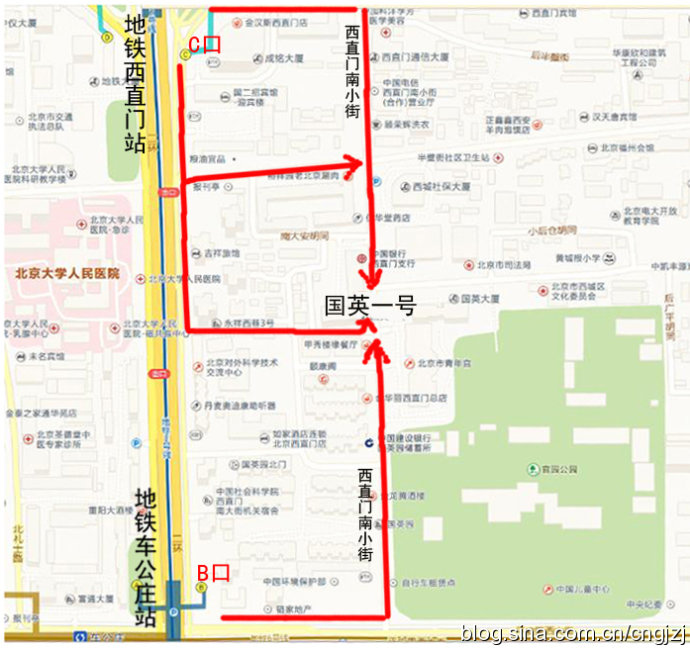
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2011年04月11日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
Drug management regulations of the medical institution
Health ministry web site
The general logistics department of the ministry of health ministry of state administration of traditional Chinese medicine
About print and distribute "the medical treatment organization pharmacy management stipulation" the notice
Health medical efforts; 11 (2011
Provinces, autonomous regions and municipalities health TingJu, administration of traditional Chinese medicine, xinjiang production and construction corps, all the military health LianQinBu, all the services and arms logistics department, the ministry of health, ZongCan three part logistics department health office, ZongCan management security ministry, zhao &industry bureau of logistics, assembly straight, military academy of sciences, the national defense university, national defense science and technology university (school) affairs department health (place), the armed forces, general logistics department, the ministry of health, logistic units directly under health departments:
In 2002, the health ministry and the state administration of traditional Chinese medicine work out medical institution "provisional regulations of the drug management (hereinafter referred to as the" interim provisions "). Implementation of the "interim provisions" in the last 8 years, various health, traditional Chinese medicine administrative departments and medical institutions in China under the efforts of medical institution, the drug management and rational drug use improved a lot. At the conclusion of the "interim provisions" around the basis of implementation, and combining the current national drug policy and drug administration medical institution of the new situation and new tasks, ministry of health and the state administration of traditional Chinese medicine and the general logistics department of the ministry of health common of the "interim provisions" has been revised, formulated the "provisions on the administration of medical institutions requirment. Hereby printed and distributed to you, please comply with. Relevant information please timely execution of YiZhengSi submitted to the ministry of public health and the state administration of traditional Chinese medicine pharmaceutical equipment YiZhengSi and logistic department bureau.
Accessories: drug management regulations of medical institution. Doc
The general logistics department of the ministry of health ministry of state administration of traditional Chinese medicine
Two itdelivers year January 30
Drug management regulations of the medical institution
The first chapter general
Article 1 in order to strengthen the drug management, and promote medical institutions, protect public drug reasonable application of health, according to the pharmaceutical administration law of the People's Republic of China law, the regulations on administration of medical institutions and the narcotics and psychotropic drugs management regulations and other relevant laws and administrative regulations.
Article 2 these provisions, the term "drug administration medical institution, it is to show medical establishment patient-centered in clinical pharmacy, based on clinical medication, the process of effective organization, implementation and management, promote the clinical science, rational drug use pharmaceutical technical service and related drugs management work.
Article 3 ministry of health and the state administration of traditional Chinese medicine is responsible for national medical institutions supervision and management of the drug management work.
Local public health administrative department at or above the county level, the Chinese medicine administrative departments shall be responsible for medical institution within their respective administrative regions supervision and management of the drug management work.
Army health administrative departments shall be responsible for the army medical institutions supervision and management of the drug management work.
Article 4 the medical institutions pharmaceutical drug management and work is an important part of the medical work. A medical institution shall, according to the regulation, set up pharmacy management organization and pharmaceutical sectors.
Article 5 the corresponding qualifications obtained according to law shall not engage in pharmaceutical professional technology personnel technology development.this work.
Article 6 medical institutions must not will be used as pharmaceuticals, medical staff or departments, department of economic distribution basis. Medical institutions and their staff must not in use of pharmaceuticals, seek improper economic interests.
The second chapter organization
Article 7 shall set up the second class above hospital drug management and therapeutics committee; Other medical institutions shall set up a pharmacy management and drug treatment, et al.
The second class above hospital pharmacy management and therapeutics committee has a senior technical position by qualifications as pharmacy, clinical medicine, nursing and hospital infection management, medical administration management staff.
Established drug management medical institution with drug treatment by the medical institutions et al pharmaceutical, medical, nursing, hospital infection, such as director of the department and the clinical departments with pharmacist, physicians above professional technical position qualifications staff.
Medical institutions as pharmacy management director with therapeutics commission (group) the chairman of the committee, pharmacy and medical director of the department appointed pharmacy management and therapeutics committee (group), deputy director of the committee.
Article 8 pharmacy management and therapeutics commission (group) shall establish and perfect the corresponding working system, by pharmaceutical department responsible for daily work.
Article 9 pharmacy management and therapeutics commission (group) responsibilities:
(a) implementing healthcare and pharmacy management and other relevant laws, regulations and rules. Audit for the drug management and pharmacy agency regulations, and supervise implementation;
(2) make this agency drug prescription set and basic drugs supply directory;
(3) promote medication relevant clinical diagnosis, guidelines and drugs for clinical use the formulation and implementation of guiding principle, monitoring, evaluation organization this medication use, proposed intervention and improvement measures, guide clinical rational drug use;
(4) analysis, evaluation drug risk and adverse drug reaction, drug damage incident and provide counselling;
(5) establish drug selection system, the audit organization this clinical departments apply for new purchases drugs, adjusting drug varieties or supply enterprises and declare matters such as hospital preparations;
(6) supervision and guidance narcotic drugs, psychotropic drugs, toxic drugs for medical use, radioactive drugs clinical use and standardized management;
(7) to medical staff on pharmacy management laws, regulations, rules and regulations and rational drug use knowledge education training; To publicize safe drug use knowledge.
Article 10 the medical department medical institution shall designate special persons, responsible and medical institutions medication related administrative affairs management.
Article 11 a medical institution shall, according to the institutions function, task, scale set corresponding pharmacy department, equipped with and provide and pharmacy department task adaptation of the professional technical personnel, equipment and facilities.
Level 3 hospital setting medicine strand, and but according to actual condition set level 2 department; Level 2 hospital Settings; pharmacy department Other medical institutions establishment pharmacy.
Article 12 for pharmaceutical department specific technology development.this drug management, service and pharmacy management, conduct patient-centered, with rational drug use is the core of the clinical pharmacy, organization participating in clinical drug therapy, pharmacists provide pharmaceutical professional technical services.
Article 13 pharmaceutical departments shall establish and perfect the corresponding working system, operating procedures and work records, and organize the implementation thereof.
Article 14 the second class above hospital pharmacy director of the department shall have the higher school pharmaceutical professional or bachelor degree or above, major in clinical pharmacy, and this professional technical position qualifications; In addition to clinic, health center, clinic, health health-care station, WeiShengZhan medical institutions other than pharmaceutical department in charge shall have college or higher school pharmaceutical professional or graduate degree in pharmacy in secondary schools, and pharmacists above professional technical position qualifications.
Chapter 3 drugs for clinical application management
Article 15 drugs for clinical use management is the medical institutions clinical diagnosis, prevention and cure disease medication, implement supervision and management of the whole process. A medical institution shall follow the safe, effective and economic rational drug use principle, respect the right of drug use patients and privacy.
Article 16 medical institutions shall, on the basis of the national system for basic drugs antimicrobial clinical guidelines and proprietary Chinese medicine clinical guidelines for the basic drugs for clinical use organization management measures, to establish and implement antibacterial drugs for clinical use grading management system.
Article 17 a medical institution shall establish the physicians, clinical pharmacists and nurses clinical treatment team, working in clinical rational drug use.
Article 18 a medical institution shall follow relevant drugs for clinical use guiding principle, a clinical pathway, clinical diagnosis, guidelines and drug manuals etc reasonable use drugs; On physician prescriptions, drug formulary suitability passmark.
Article 19 medical institutions shall be equipped with clinical pharmacist. The clinical pharmacists in clinical drug therapy should be full-time work, education, for patients with medication guide use medicine safely.
Article 20 a medical institution shall establish clinical medication monitoring, evaluation and supernormal warning system for drugs for clinical use, security and efficiency and economy analysis and monitoring, evaluation, the implementation of prescription and drug formulary comment on and intervention.
Article 21 a medical institution shall establish adverse drug reaction, medication errors and drug damage the event monitoring report system. Medical institutions clinical departments find adverse drug reaction, medication errors and drug damage incident, should actively cure patients, pharmaceutical department report immediately to observing and recording, and completes. A medical institution shall, in accordance with the relevant state provisions to related department report adverse drug reaction, medication errors and drug damage at the county level shall immediately report to the local events in the administrative department of public health reports.
Article 22 medical organizations shall, in combination with clinical and medication, developing clinical pharmacy and pharmaceutical research work and provide necessary working conditions, formulate the corresponding management system, and strengthen leadership and management.
The fourth chapter elixir management
Article 23 a medical institution shall, according to the national basic drugs directory ", "prescription, the measures for the administration of integrating", "national prescription drug purchasing and supply the quality control standard for the institution of the pharmaceutical prescription set" and "the catalogue of basic drugs, preparation of drug supply purchase plan, according to the rules, buy drugs.
Article 24 a medical institution shall formulate organization this drug purchasing process; Establish and perfect the drug cost accounting and accounting management system; Strict execution drug buy check and acceptance system; May not buy and use of drugs is not in conformity with the provisions.
Article 25 the medical institution shall be used by drug clinical pharmaceutical department unified purchase and supply. The pharmacy management and therapeutics commission (group) examine and verify agrees with you can purchase, nuclear all-around-doctors dispensing, this professional required radioactive drugs. Other department or agency shall not be engaged in drug procurement, dispensing activities and may not in clinical use non-pharmaceutical department purchase and supply of the drug.
Article 26 a medical institution shall compile and execute drug storage system, regular maintenance of inventory drugs with quality inspection. The storage conditions and management YaoPinKu shall conform to the quality control standard for pharmaceutical purchasing and supply the relevant regulations.
Article 27 chemical, biological products, traditional Chinese medicine and Chinese medicine yinpian shall be respectively storage, classified positioning for storage. Inflammable, explosive, corrosive risk should be another such a drug store, and set the warehouse separate the necessary safety facilities, work out relevant work system and emergency plan.
Narcotic drugs, psychotropic drugs, toxic drugs for medical use, radioactive drugs and other special management medicines, shall, in accordance with the relevant laws, regulations and rules of the relevant provisions of the management and supervision to use.
Article 28 pharmaceutical professional technology personnel should strictly in accordance with the pharmaceutical administration law, the prescription drug dispensing management measures of quality control standard, such laws, regulations, rules and regulations and technical operation procedures and carefully examine and verify the prescription medication by prescribing, or adjust the suitability after drug dispensed review. When issued shall be informed patient drug usage and dosage and precautions, guiding patients rational drug use.
For ensuring patients drug safety, in addition to the drug quality, the reason is sent out, not drug for replacement.
Article 29 DiaoJiShi medical institution shall be a big drug this window or counter type hair medicine. DiaoJiShi hospital (ward) drugs according to daily doses of injection of oral preparations dispensed, and a single dose of drug dispensing dispensed.
Parenteral nutrients and drugs endangering drugs shall centralize and intravenous deployment supply.
Article 30 medical institutions according to the clinical need to build intravenous drug control center (room), centralize and allocate supply. Intravenous drug control center (room) shall conform to the vein quality control standard drug concentration deployment by the local districts, the health administrative department at or above the level of technology review, acceptance, organization qualified rear can focus allocate intravenous drug use. After intraenous drug control center (room) beyond medicine, the reference vein deployment vein quality control standard drug concentration deployment implementation.
Medical institutions create vein drug control center (room) shall be submitted to the provincial health administrative department for record.
Article 31 the medical treatment organization product management in accordance with the pharmaceutical administration law and its implementing regulations and other relevant laws and administrative regulations provisions.
Chapter 5 pharmaceutical professional technology personnel allocation and management
Article 32 pharmaceutical professional technology personnel medical institution in accordance with the relevant provisions of obtaining the corresponding pharmaceutical professional technical position qualifications.
Medical institutions direct contact with drugs shall be conducted annually pharmaceutical personnel, health inspection. Suffering from contagious diseases or any other disease which may contaminate the medicine shall not be engaged in direct contact with drugs.
Article 33 pharmaceutical professional technology personnel medical institution shall not be less than this institution 8% of health professionals. Establish intravenous drug control center (room), the medical institution shall, according to the actual needs to be increased number of pharmaceutical professional technical personnel.
Article 34 a medical institution shall, according to the agency, task, scale is equipped with the appropriate number of clinical pharmacists third-level hospital clinical pharmacists, not less than 5, the secondary hospital clinical pharmacist no less than three hundred.
The clinical pharmacists in clinical pharmacy shall have the higher school undergraduate professional or pharmaceutical professional qualifications, and above shall pass standardization training.
Article 35 a medical institution shall strengthen the cultivation of pharmaceutical professional technical personnel, evaluation and management, formulate training plan, organize pharmaceutical professional technology personnel to participate in after the graduation standardization training and continued medical education, will complete the training education credits and receive medical continue, as pharmaceutical professional technology personnel evaluation, promotion and professional technical position qualifications and professional post appointment one of the conditions.
Article 36 the pharmacists work responsibilities: medical institution
(a) shall be responsible for drug purchasing and supply, prescription or drug use, drug dispensing and noncompliance audit drug concentration deployment and venous hospital preparations configuration, guiding ward (area) nurse please bring, use and management drugs;
(2) participate in clinical drug therapy, for individualized medicine treatment plan, carry out the design and implement of pharmaceutical rounds for patients with provide pharmic professional and technical services;
(3) attend rounds, consultation, case discussion and difficulty, critically ill patients with medical treatment, the collaborative doctors do drug use in clinical drug therapy selection, opinions or advice, and doctors adjustment of drug therapy is responsible for common;
(4) develop antibacterial drugs for clinical use monitoring, implement prescription comment on and supernormal warning, promote drug reasonable use;
(5) carry out drug quality monitoring, drug serious adverse reactions and drug damage of collecting, sorting, reports etc;
(6) master and clinical drug related drug information, offering medical information and pharmacy consulting services, to publicize rational drug use knowledge;
(7) combined with clinical drug treatment practice, carries on the pharmaceutical clinical application research; Seeding drug use evaluation and drug clinical application research; Participate in new drug clinical trials and drug safety and efficacy listed after monitoring;
(8) other and relevant professional technology hospital pharmacy.
The sixth chapter supervision and management
Article 37 where health, traditional Chinese medicine at or above the county level shall strengthen the administrative department of medical institutions of drug administration supervision and management.
Article 38 the medical institutions must not use non-pharmaceutical professional technical staff engaged in pharmaceutical professional technical work or hire, director of the department of the pharmacy.
Article 39 the medical establishment occurrence of the following circumstances, the county-above health, traditional Chinese medicine administrative department to make corrections, criticized, give warning; For the leading persons directly in charge and other persons directly responsible for it shall be given relegation, removed, fired etc. Punishment:
(a) drug management organization has not been established, pharmacy management and technology development.this caused confusion, work safety hazard and serious adverse health consequences;
(2) not in accordance with these regulations, equipped with professional and technical personnel, establish pharmaceutical clinical pharmacists system, not the serious problem, and the rational use of drugs in the adverse impact;
(3) does not perform relevant drug quality management norms and regulations, lead to drug quality problems or medication errors, causing medical security hidden danger and serious adverse consequences;
(4) non-pharmaceutical department purchase and use in drug dispensing, or agents activities;
(5) will use of pharmaceuticals, as a personal or departments, department of economic distribution basis, or in the use of pharmaceuticals, seek improper interest;
(6) in violation of these provisions of other provisions and serious consequences.
Article 40 medical institution violates drug management relevant laws, regulations and rules, according to its plot where by public health administrative department at or above the county level shall be dealt with according to law.
Article 41 local health at or above the county level, the Chinese medicine administrative department shall regularly drug management of medical institutions conduct supervision and examination.
Article 42 administrative department of public health, the Chinese medicine functionaries of drug administration of medical institutions, when conducting supervision and inspection shall produce their certificates. Check the medical institutions shall cooperate with and truthfully report the situation to them and provide the necessary materials, and may not refuse or obstruct, conceal.
Chapter 7 remarks
Article 43 the provisions in the following words meaning:
Clinical pharmacy: refers to the combined with clinical pharmacists, directly facing the patients to the patient as the center, the research and practice of clinical drug therapy, improve the comprehensive application level of drug treatment subject.
Clinical pharmacist: refers to the system for pharmaceutical professional knowledge based, and have certain medical and relevant professional knowledge and skills, direct participation in clinical medicine, to promote the rational application and protect patients drug medication safety pharmaceutical professional and technical personnel.
Harm drug: refers to can produce occupational exposure and dangerous or harmful drug, namely with genetic toxicity, carcinogenicity of teratogenic sex, or, birth damage at low doses and produces serious organs or other aspects of drugs, including toxic chemotherapy drugs and cytotoxic drugs.
Drug damage: it is to because drug quality does not accord with the national drug standard for patients caused the damage.
Medication errors: refers to the whole drug in clinical use qualified appear in any of the drug can prevent, improper.
Article 44 the management of traditional Chinese medicines into ready-to-use forms of medical institutions, in accordance with the hospital Chinese medicine yinpian control criterions execution.
Article 45 the clinic, health center, clinic, health WeiShengZhan don't set station and drug management organization and pharmacy department designated by orgnaization controller, medical staff drug work for.
TCM clinic, national medical clinics don't set pharmacy management organization and pharmacy department, traditional Chinese medicine and the folk medicine by specialized technical personnel responsible for drug work.
Article 46 the provisions shall March 1, 2011 promulgation. "The medical treatment organization pharmacy management provisional regulation" (who sends [2002] medical # 24) shall be repealed simultaneously.