中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
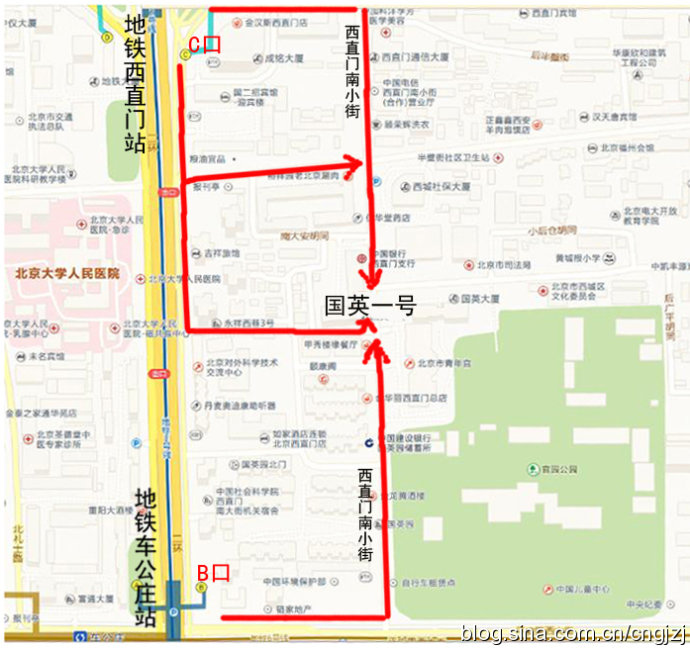
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2011年05月31日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
The adverse drug reaction monitoring report
and revision of the measures for the administration of release
The newly revised report and adverse drug reaction monitoring to manage the way "(hereinafter referred to as" new revision of the regulations "), and was formally promulgated on 1 July implementation.
The adverse drug reaction reports and monitoring to manage the way "(hereinafter referred to as" the way "") is our country develop adverse drug reaction monitoring work important legal basis. "Method" revision is the drug agency to implement the scientific development concept and medical health system reform requirements, further attention to protect public people's livelihood, safe drug use another major move, its implementation will further promote adverse drug reaction monitoring, the launch of the various work for safeguarding the drug use safety built an effective barrier.
In 2004 the regulations promulgated) is China's first adverse drug reaction reports and monitoring management of administrative regulations, since the implementation of, our country from adverse drug reaction monitoring work report and rapid development, monitoring system further perfect, the report quantity and quality enhances unceasingly. But as the drug regulatory changes and adverse drug reaction monitoring work thorough, "method" also exposed some shortcomings, such as: local adverse drug reaction monitoring institutions and duties already can not adapt to the current set of drug safety supervision needs; A pharmaceutical production enterprise the first owner reflect enough; Statisticians, omission phenomenon remains; For serious adverse drug investigation and handling, and requests the enterprise to already listed pharmaceuticals lack of clear provisions such as safety researches. To solve these problems, the ministry of health and the state food and drug administration to "method", perfecting and complements to modify that makes it more accord with current and future a period of time inside of regulatory requirements. The newly revised method "eight chapters, including general, 67 of duties, reports and disposal, key monitoring, evaluation and control, information management, legal responsibility and bylaws. The newly revised method "further specify the province following regulators and adverse drug reaction monitoring agency responsibilities and standardizes the report process and requirements, added to serious adverse drug reaction, group drug adverse events investigation to verify the evaluation requirements, increased" drugs, "key monitoring requirements of production enterprises carrying out monitoring work initiative forward more explicit and higher requirements.
The food and drug administration required food and drug supervision departments at all levels should strengthen adverse drug reaction monitoring work report and the leadership, carefully pays special attention to the new castigatory "way" learning, publicity and carry out the work; Strengthening primary adverse drug reaction monitoring institutions and monitoring capability construction, improve the adverse drug reaction information collection, reporting, analysis, evaluation and processing capacity; Establish and perfect the adverse drug reaction monitoring systems and procedures, refine monitoring, the detailed rules for the implementation of the operating process and working standard to improve monitoring of institutionalized, standardization and scientific level; And the public health administrative department close coordination and cooperation, strengthen drug group adverse events report, survey, processing work and ensure the works forceful, orderly and effective, ascension drug safety warning ability and level.
The food and drug administration required pharmaceutical production, trading enterprises and medical organizations active surveillance, reporting, analysis and evaluation of adverse drug reaction, especially the pharmaceutical production enterprise shall actively strengthening adverse drug reaction monitoring work and actively taking risk management measures to control drug risk.
【 relevant link 】
The adverse drug reaction reports and monitoring to manage the way "(health ministry make the 81).