中国民间中医医药研究开发协会国际针灸合作委员会
办公地点现在已经搬迁至西城区西直门南小街国英园一号楼824室,
同时为方便大家联系,固定电话已经变更
新号码010—58562339。特此通知。
地址:北京西城区西直门南小街国英园一号楼824室
邮编:100035
电话:010-58562339
传真:010-58562339
邮箱:cngjzj@163.com
网站(点击网址直接链接↓):http://www.cngjzj.com/
博客(点击网址直接链接↓):http://blog.sina.com.cn/cngjzj
交通路线图 (点击观看大图)
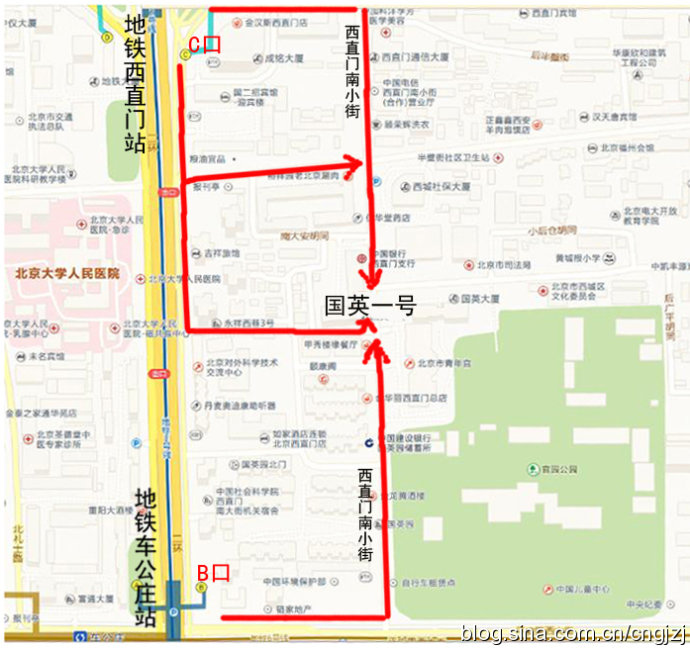
从首都机场乘坐机场专线,在东直门站下车换乘地铁2号线开往西直门方向,在西直门站 C 口出站:
1、沿西直门内大街向东直行100米,右拐到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
2、向南直行50米,绕过 国二招宾馆 沿着中大安胡同向东到西直门南小街,向南步行到丁字路口即到国英园1号楼楼下。
从首都机场内乘坐机场直达西单的大巴,在西单站下车,乘坐出租车到西直门南小街国英园1号楼。
公交官园站:107路,运通106路
公交西直门南:387路,44路,800内环,816路,820内环,845路
地铁车公庄:地铁二号线
地铁西直门:地铁二号线
公交车公庄东:107路,118路,701路
公交车公庄北:209路,375路,392路
 您现在的位置是:
首页 >>
Regulations >>
Regulations
您现在的位置是:
首页 >>
Regulations >>
Regulations
2011年06月16日
 复制链接
复制链接
 打印
打印
 大 中 小
大 中 小
The measures for the management of medical equipment recall (trial implementation), ministry of health (no. 82)
The health ministry
Ministry of public health of the People's Republic of China
No. 82
The measures for the management of medical equipment recall (try out) "already in June 2010 and the health ministry by the ministerial meetings, now be released, since July 1, 2011 and implementation.
Department long subtends Chen
○ 2 by May 20,
Measures for the management of medical equipment recall (for trial implementation)
The first chapter conditions
Article 1 in order to strengthen the supervision and administration of medical devices, safeguard human health and life safety, according to the medical equipment supervision and management regulations ", "the state council on strengthening the supervision and management of food safety of products such as the special provisions of the present measures.
Article 2 in the People's Republic of sales of medical devices and the supervision and management, the recall of application of this method.
Article 3 of these measures, the term "medical equipment recall, it is to show medical device manufacturing enterprise in accordance with the prescribed procedures for its already listed sales of the defect of a category, model or batches of product take warning, checked, repaired, to label, modification and improvement of the manual, software upgrade, replaced, and withdraw, destroyed, eliminate defect behavior. Way
Article 4 the term "defects, it is to show medical equipment under normal use existence could endanger human body health and life safety of unreasonable risk.
Article 5 medical device manufacturing enterprise is to control and eliminate the defects of the main body, the production of products shall be responsible for security.
Article 6 medical device manufacturing enterprise shall, in accordance with the provisions of the measures to establish and perfect the medical equipment recall system, collect medical equipment safety of relevant information, the defects of may of medical devices for investigation, evaluation, timely recalled the defects of medical apparatus and instruments.
Medical equipment management enterprises, use the unit shall assist the medical device manufacturing enterprise to perform obligations in accordance with the plan, the recall recall the requirements of communication, timely feedback medical equipment recall information, control and take back the defects of medical apparatus and instruments.
Article 7 medical equipment management enterprises, use the unit found its operation, use of medical devices shall be the defects, immediately suspend sales or use the medical equipment, promptly notify the medical device manufacturing enterprise or to the local suppliers, and of the provinces, autonomous regions, municipalities directly under the central government pharmaceutical supervisory and administrative department report; Use the unit for medical institutions shall at the same time, also to the local provinces, autonomous regions, municipalities directly under the central government, and report to the administrative department of health.
Medical equipment management enterprises, use of units of the provinces, autonomous regions, municipalities directly under the central government where the pharmaceutical supervisory and administrative departments shall, after receiving the report promptly inform medical device manufacturing enterprise of provinces, autonomous regions, municipalities directly under the central government where the pharmaceutical supervisory and administrative department.
Article 8 the recall of medical equipment production enterprise, import medical equipment in the overseas manufacturers designated agent in China of the provinces, autonomous regions, municipalities directly under the central government where the pharmaceutical supervisory and administrative departments shall be responsible for the supervision and administration of medical devices recall work, other provinces, autonomous regions, municipalities directly under the central government pharmaceutical supervisory and administrative departments shall cooperate and assist the well in the local medical equipment recalled the related work.
The food and drug administration supervision and the national medical equipment recalled management work.
Article 9 the state food and drug administration of provinces, autonomous regions, municipalities directly under the central government and the pharmaceutical supervisory and administrative departments shall establish medical equipment recall information reporting and open system in time, to the public health administrative department report relevant information, take effective way to the public of the defect of medical equipment information and medical equipment recall.
The second chapter medical equipment investigation and evaluation of the defects
Article 10 medical device manufacturing enterprise shall set up and perfect the quality management system for medical devices and medical device adverse event monitoring system, collect, record of medical devices quality problem and medical device adverse event information, to collect information analysis of medical devices, possible defects investigation and evaluation.
Medical equipment management enterprises, use the unit shall cooperate with medical device manufacturing enterprise related medical equipment defect of the survey, and provide the relevant information.
Article 11 the medical device manufacturing enterprise shall, in accordance with the provisions in time will collect the medical device adverse event information to the pharmaceutical supervisory and administrative department report, the pharmaceutical supervisory and administrative department of medical devices can be adverse events information or possible defects analysis and investigation, medical device manufacturing enterprise, business enterprise, use the unit shall provide assistance.
Article 12 for medical equipment defects of evaluation content include:
(a) in the use of medical equipment process whether happened failure or damage;
(2) in the existing use environment would cause harm, whether there is a scientific literature, research, and related tests or validation to explain the reason of injuries;
(3) involved in the damage range and the crowd characteristics;
(4) to the health of human body of the damage the degree;
(5) damage the probability of occurrence in a;
(6) the short term and the long term damage occurred consequences;
(7) the other may cause harm to human body factors.
Article 13 according to medical devices to the severity of the defects, medical equipment recall is divided into:
(a) level 1 recalled: use the medical equipment may have caused serious health hazard or;
(2) level 2 recalled: use the medical equipment may have cause temporary or or reversible health hazard;
(3) level 3 recalled: use the medical devices are less likely to cause harm but still recalled.
Medical device manufacturing enterprise shall, according to the classification and recall medical devices, sale and use of scientific design plan and implement it. The recall
The third chapter voluntarily recalled
Article 14 the medical device manufacturing enterprise in accordance with article 10 of the present measures, article 12 requirements researched review found that the defect of medical devices, it shall immediately decided to recall.
Import medical equipment in the overseas manufacturers outside in the implementation of the medical equipment recall, it shall notify the designated agent in China of timely report the state food and drug administration; In the territory of the recall, by its designated agent in China in accordance with the provisions of the present measures in charge of specific implementation.
Article 15 the medical device manufacturing enterprise to make medical equipment recall decision, level 1, level 2 days in the recall recall in 3 days, level 3 recalled on 7 days notice to the relevant medical devices, business enterprise, use the unit or inform the user.
Recall notice shall at least include the following content:
(a) recalled medical equipment name, batch basic information;
(2) recalled reasons;
(3) recalled requirements: such as immediately suspend sales and use the products, will recall to related business enterprise or forwarding use unit and so on;
(4) the recall the processing means of medical devices.
Article 16 medical device manufacturing enterprise to make medical equipment recall decision, it shall immediately notify in writing the provinces, autonomous regions, municipalities directly under the central government where the pharmaceutical supervisory and administrative departments, and in 5 days to fill in "the medical equipment recall of report form (see appendix 1), will investigate the evaluation report and plan to recall submitted at the same time of the provinces, autonomous regions, municipalities directly under the central government where the pharmaceutical supervisory and administrative departments for the record.
Provinces, autonomous regions, municipalities directly under the central government pharmaceutical supervisory and administrative departments shall make the recall of the relevant report in time the state food and drug administration.
Article 17 study report shall include the following contents:
(a) the recall of the specific conditions of the medical devices, including name, batch basic information;
(2) the implementation of the recall cause;
(3) study results;
(4) recalled grading.
Recall plan shall include the following content:
(a) medical equipment production and sales to the number of recalled;
(2) the specific content of the recall measures, including implementation of the organization, the scope and duration, etc.;
(3) the recall of the information released way and the area;
(4) recalled the expected effect;
(5) medical devices after the recall processing measures.
Article 18 the pharmaceutical supervisory and administrative department may according to the actual situation of the organization of experts to medical device manufacturing enterprise of the recall plans to submit assessment, and think medical device manufacturing enterprise measures to eliminate the defects can not effectively, shall require medical device manufacturing enterprise to improve the level and expand the recall recall scope, shorten the time or change the recall recalled products such as handling more effective measures.
Article 19 the medical device manufacturing enterprise to report of the recall plan changes, it shall be timely report pharmaceutical supervisory and administrative departments for the record.
Article 20 the medical device manufacturing enterprise in the process of implementing the recall, according to recall plan shall be periodically to the pharmaceutical supervisory and administrative department for the recall plan implementation report (see form 2), the report recalled plan.
Article 21 the medical device manufacturing enterprise to recall the processing of medical devices shall have the detailed record, and to medical device manufacturing enterprise of provinces, autonomous regions, municipalities directly under the central government where the pharmaceutical supervisory and administrative department report. For through the warning, checked, repaired, to label, modification and improvement of the manual, software upgrade, replaced, and destroy the way and can eliminate the defects in the product is located, can accomplish these behavior. The need to destroy, shall be destroyed in to pharmaceutical supervisory and administrative department under the supervision of destruction.
Article 22 the medical device manufacturing enterprise shall, after the recall in complete on recalled, and evaluate the effects in the recall within 10 days after the completion of the pharmaceutical supervisory and administrative departments of the state council for medical equipment recall summary report.
Article 23 the pharmaceutical supervisory and administrative departments shall receive the summary report within ten days of date of examination of the report, and evaluate the effects of recall. Review and appraisal conclusion by the written form notice shall be medical device manufacturing enterprise and send duplicates the public health administrative department.
After examination and evaluation, think recall does not completely, have yet to effectively eliminate the defects, pharmaceutical supervisory and administrative department shall request the medical device manufacturing enterprise to recall.
The fourth chapter shall be ordered to recall
Article 24 the pharmaceutical supervisory and administrative department investigation, evaluation of these measures say that there are "mentioned in article 4 of the defect, medical device manufacturing enterprise shall recall medical equipment and did not voluntarily recalled, it should be ordered to medical device manufacturing enterprise recalled medical equipment.
When necessary, the pharmaceutical supervisory and administrative departments shall request the medical device manufacturing enterprise, business enterprise and use the unit immediately suspend sales or use and inform the user immediately suspend the use of the medical equipment.
Article 25 the pharmaceutical supervisory and administrative departments shall be ordered to make decision, it shall be ordered to recall recall notice to medical device manufacturing enterprise or import medical device manufacturing enterprise of domestic agents, and the notice includes the following content:
(a) the recall of the specific conditions of the medical devices, including name, batch basic information;
(2) the implementation of the recall cause;
(3) study results;
(4) requirements, including range and recall quantity, etc.
Article 26 the medical device manufacturing enterprise received the notification shall be ordered to recall, in accordance with the provisions of article 15 and article 16 by notice medical equipment management enterprise and use the unit or inform the user, formulate, submit the recall, and organize the implementation plan.
Article 27 the medical device manufacturing enterprise shall, in accordance with article 19 of the present measures, of article 20, article 21, the provisions of article 22 of the pharmaceutical supervisory and administrative department to report the related information of medical equipment recall, recalling medical equipment further treatment.
Pharmaceutical supervisory and administrative departments shall, in accordance with article 23 hereof to medical device manufacturing enterprise submit medical equipment recall summary report for review and evaluate the effects of the recall, promptly inform the public health administrative department. After examination and evaluation, think recall does not completely, have yet to effectively eliminate the defects, pharmaceutical supervisory and administrative department shall request the medical device manufacturing enterprise to recall.
Chapter 5 legal responsibility
Article 28 the pharmaceutical supervisory and administrative department confirmed that medical device manufacturing enterprise in violation of laws, regulations and rules of the law causing listed medical equipment defects, shall be given administrative punishments according to law, but this enterprise has taken active recall measures to eliminate or reduce the harmful consequences, in accordance with the provisions of administrative punishment law, be given a lighter or mitigated punishment; Minor illegal act and timely correcting and causes no harmful consequences, no punishment.
Medical device manufacturing enterprise recall of medical devices, not exempt from the law shall be liable for the other legal responsibility.
Article 29 the medical device manufacturing enterprise, in violation of the provisions of medical devices, found defects and not voluntarily recalled medical devices shall be ordered to recall, medical equipment, impose medical devices shall be recalled a nominal value of three times the fine; Serious consequences, the original license issuing department due to the product registration certificate for a medical device until revoked, the medical device manufacturing enterprise license.
Article 30 medical device manufacturing enterprise in violation of the provisions of article 24 of the present measures, refused to recall the medical equipment, medical equipment should be recalled at a nominal value of three times the fine; Serious consequences, the original license issuing department due to the product registration certificate for a medical device until revoked, the medical device manufacturing enterprise license.
Article 31 the medical device manufacturing enterprise has one of following state, be warned, to make corrections within a time limit, and shall also be fined 30000 yuan of the following:
(a) violates these measures prescribed in article 15, not in time will recall the decision of the medical devices to medical equipment management enterprise notice, use the unit or inform the user;
(2) violates these measures article 18, paragraph 2 of article 23, paragraph 2 of article 27, not according to the pharmaceutical supervisory and administrative departments requirement to take corrective measures or recalled to the medical equipment;
(3) violates these measures, not the provisions of article 21 of the recall of medical devices do not deal with detailed records or to the pharmaceutical supervisory and administrative departments of the report.
Article 32 medical device manufacturing enterprise has one of following state, be warned, shall be ordered to make corrections within; If no correct, place to 30000 yuan:
(a) not prescribed in the measures establish medical equipment recall system;
(2) refused to assist pharmaceutical supervisory and administrative department of investigation;
(3) fails to submit the provisions of the medical device recall report form ", investigate assessment report and recalled plan, medical equipment recall plan and summary report;
(4) change plan, without reporting the recall the pharmaceutical supervisory and administrative departments for the record.
Article 33 medical equipment management enterprises, use the unit violates these measures prescribed in paragraph 1 of article 7, shall be ordered to stop the sale and use of existing defects of medical devices, also be fined not more than 1000 yuan of above 30000 yuan of the following amerce; Serious consequences, the original license issuing department due to the medical equipment management enterprise licence ".
Article 34 the medical equipment management enterprises, use the unit refused to cooperate with the concerned medical equipment defects investigation, refused to assist medical device manufacturing enterprise recall of medical devices, be warned, rectification shall be ordered; Refused to put it right, the place to 30000 yuan.
Article 35 the pharmaceutical supervisory and administrative departments and their staff not to perform its obligations hereunder or abuse of power, according to relevant laws, rules and regulations, provisions of processing.
Chapter 6 remarks
Article 36 the recall of medical equipment has implantation in the human body, medical device manufacturing enterprise shall and medical institutions and patients, according to negotiate the different reasons and puts forward the recall to the patient's treatment Suggestions and measures should be taken in the plan.
Article 37 the recall of medical devices to patients, patients can cause damage to the production enterprise, can also claim for compensation to the medical equipment management enterprises, use the unit request for compensation. Patients to medical equipment management enterprises, use the unit claims for compensation for medical equipment management, enterprise, use the unit after paying compensation, shall have the right to recover the responsible production enterprise.
Article 38 the measures from July 1,.